No CrossRef data available.
Published online by Cambridge University Press: 27 September 2024
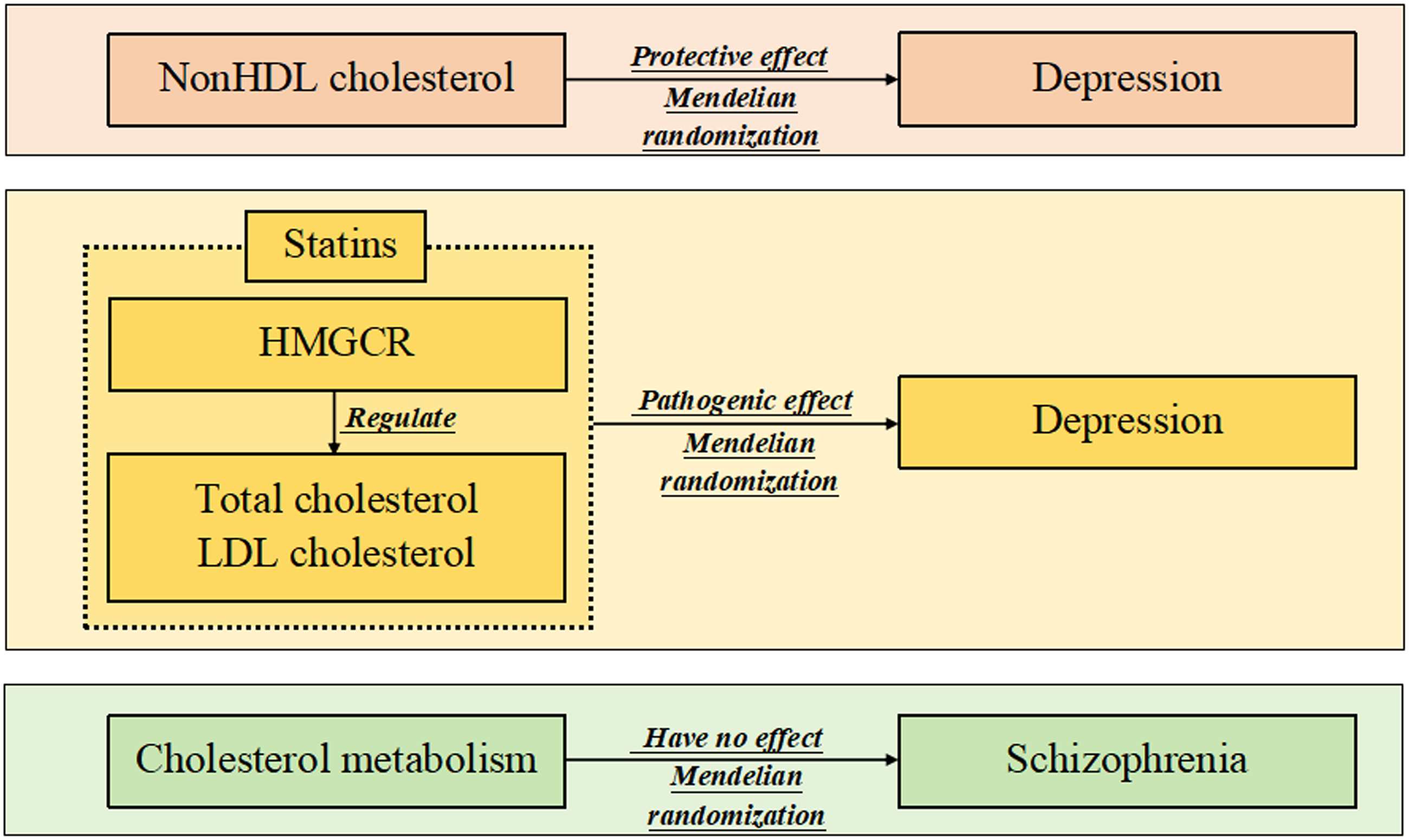
Some observational studies have unexpectedly reported the association of cholesterol metabolism with mental and psychological disorders, but a firm conclusion has not been drawn. The aim of this study was to further investigate the effects of peripheral cholesterol traits and cholesterol-lowering therapy on depression and schizophrenia using a Mendelian randomisation approach.
Instrumental variables meeting the correlation, independence and exclusivity assumptions were extracted from one genome-wide association study for predicting total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and nonHDL cholesterol. Instrumental variables for total cholesterol and LDL cholesterol were also adopted to predict statin use (a type of cholesterol-lowering drug); these instrumental variables should not only satisfy the above assumptions but also be close to 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR, the target gene of statins) on the chromosome. Three methods (including inverse variance weighted) were used to conduct causal inference of the above exposures with depression and schizophrenia. Sensitivity analyses were performed to assess horizontal pleiotropy.
Higher levels of peripheral nonHDL cholesterol were nominally associated with a decreased risk of depression (P = 0.039), and higher levels of HMGCR-mediated total cholesterol and LDL cholesterol were nominally related to a decreased risk of depression (P = 0.013 and P = 0.028, respectively). Moreover, these cholesterol traits cannot affect the risk of schizophrenia. Sensitivity analysis did not reveal any horizontal pleiotropy.
The study provided some interesting, but less sufficient, evidence that nonHDL cholesterol may have a protective effect on depression, and lowering cholesterol using statins might increase the risk of the disease.