Summations
Bipolar disorder (BD) may be a disorder of the entire body and not just the brain.
Cannabis can affect age of onset of BD, severity, and the number of affective episodes. Both arachidonic acid (AA) and inflammatory mediators can play a part in the pathophysiology of BD.
A proposed new treatment strategy of selective activation of cannabinoid receptor 2 (CB2) and antagonism of cannabinoid receptor 1 (CB1) may alleviate the symptoms of BD and should be rigorously explored.
Perspectives
1. The role of endocannabinoid system (ECS) in mood control and BD is suggested based on scant available data; however, data from these studies warrant more comprehensive studies, which are essential to empirically test whether ECS is involved in mood and to determine the mechanisms of action for ECS in regulation of affect. Although there is a surge in research on CBs, the possible role of the ECS in neuropsychiatric disorders, especially BD, has almost been neglected. Thus, there is an unmet need for conducting more clinical investigations of agents which affect the ECS in bipolar patients in order to pursue other treatment strategies than those currently available to normalise mood.
2. The lipophilic nature of the CB ligands, as well as their long biological half-life, confers the ability to cross the blood–brain barrier easily. Further, their high therapeutic index reduces the risk of overdose in BD patients which is highly relevant in the management of the BD population.
3. Selective activation of CB2 could provide mood stabilisation by suppressing AA turn over, which is a mechanism common to various types of currently available mood stabilisers.
Introduction
Bipolar disorder (BD) is a debilitating lifelong neuropsychiatric disorder that is characterised by unstable high and low episodes of moods which swing between the extremes of (hypo)mania and depression. Extremes in the mood state tend to alternate in a cycle and are usually punctuated by periods of remission (Oswald et al., Reference Oswald, Souery, Kasper, Lecrubier, Montgomery, Wyckaert, Zohar and Mendlewicz2007; Fagiolini et al., Reference Fagiolini, Forgione, Maccari, Cuomo, Morana, Dell’Osso, Pellegrini and Rossi2013; Arjmand et al., Reference Arjmand, Behzadi, Stephens, Ezzatabadipour, Seifaddini, Arjmand and Shabani2017). BD takes a heavy toll and influences most aspects of the life of those who suffer from this disease with negative effects on their personal relationships, their social interactions, their performance in the workplace, and their ability to pursue educational goals (Leclerc et al., Reference Leclerc, Mansur and Brietzke2013).
Lithium is a commonly used approach in the pharmacological toolbox for the management of BD, which remains popular some 70 years after its introduction. Along with this gold standard treatment approach, other mood stabilisers including sodium valproate, carbamazepine, lamotrigine, and atypical antipsychotics, such as quetiapine and olanzapine, have been broadly used to alleviate the symptoms of both (hypo)mania and depression (Ashton et al., Reference Ashton, Moore, Gallagher and Young2005; Shorter, Reference Shorter2009; Rapoport, Reference Rapoport2014; Sportiche et al., Reference Sportiche, Geoffroy, Brichant-Petitjean, Gard, Khan, Azorin, Henry, Leboyer, Etain, Scott and Bellivier2016). Despite possessing a wide range of compounds with diverse mechanisms of action with which to ameliorate the acute affective symptoms and preclude episodic relapse, BD is sometimes inadequately treated (Ashton et al., Reference Ashton, Moore, Gallagher and Young2005).
Both serotonergic (Burokas et al., Reference Burokas, Martín-García, Gutiérrez-Cuesta, Rojas, Herance, Gispert, Serra and Maldonado2014) and dopaminergic transmissions play a prominent role in the pathophysiology of neuropsychiatric disorders, and the fact that these systems are modulated by the endocannabinoid system (ECS) suggests examination of this system to potentially facilitate developing a new target to better control mental illnesses including BD (Van Der Stelt & Di Marzo, Reference Van Der Stelt and Di Marzo2003; Arjmand et al., Reference Arjmand, Behzadi, Stephens, Ezzatabadipour, Seifaddini, Arjmand and Shabani2017; Ashok et al., Reference Ashok, Marques, Jauhar, Nour, Goodwin, Young and Howes2017).
The ECS is comprised of cannabinoid (CB) receptors, endogenous lipid ligands, as well as enzymes which are in charge of both endocannabinoid synthesis and degradation that together play a neuromodulatory role in the central nervous system (CNS) (Arjmand et al., Reference Arjmand, Vaziri, Behzadi, Abbassian, Stephens and Shabani2015; Lu & Mackie, Reference Lu and Mackie2016). CB receptors are in a class of G protein-coupled receptors and are divided into two main subtypes, CB receptor types 1 and 2 (CB1 and CB2, respectively) (Arjmand et al., Reference Arjmand, Vaziri, Behzadi, Abbassian, Stephens and Shabani2015; Lu & Mackie, Reference Lu and Mackie2016). CB1 receptors are ubiquitous in the CNS and are located pre- and postsynaptically on neurons, whereas CB2 receptors were originally thought to occur only in the periphery and to be located on monocytes, macrophages, B cells, and T cells, where they played a role in immune system functions (Arjmand et al., Reference Arjmand, Vaziri, Behzadi, Abbassian, Stephens and Shabani2015; Lu & Mackie, Reference Lu and Mackie2016). However, recent evidence indicates that CB2 receptors are present in the brain, albeit to a lesser extent than the CB1 receptors (Atwood & Mackie, Reference Atwood and Mackie2010). These receptors have been detected in microglia and therefore play a role in the brain’s immune system, and there are reports showing that in pathological conditions their presence is enhanced (Viscomi et al., Reference Viscomi, Oddi, Latini, Pasquariello, Florenzano, Bernardi, Molinari and Maccarrone2009; Lu & Mackie, Reference Lu and Mackie2016).
The ECS and signaling pathways to which the system is coupled have emerged to be of key importance in the regulation of processes underlying executive function, including emotion, reward, learning, and memory (Vinod & Hungund, Reference Vinod and Hungund2006; Roche & Finn, Reference Roche and Finn2010; Haghani et al., Reference Haghani, Shabani, Javan, Motamedi and Janahmadi2012; Wei & Piomelli, Reference Wei and Piomelli2015; Abbassian et al., Reference Abbassian, Esmaeili, Tahamtan, Aghaei, Vaziri, Sheibani, Whalley and Shabani2016). The ubiquitous neural presence of CB receptors, particularly CB1 receptors, means they are present in brain areas which are involved in mood disorders such as the hippocampus, cerebellum, basal ganglia, and cortex. The localisation of the ECS system, when coupled with the well-known psychoactive properties of Cannabis sativa, has encouraged a dramatic peak of research to truly understand the role this intricate system plays in mental illnesses (Vinod & Hungund, Reference Vinod and Hungund2005, Reference Vinod and Hungund2006; Carvalho & Van Bockstaele, Reference Carvalho and Van Bockstaele2012; Esteban & García-Sevilla, Reference Esteban and García-Sevilla2012; Hillard et al., Reference Hillard, Weinlander and Stuhr2012).
Aims of the study
The aim of this current mini review is to provide evidence that dysfunction of the ECS could play a role in the course of BD and via examination of a range of studies including clinical work and molecular investigations to challenge the idea of whether this system represents an avenue to pursue the development of another mechanistic treatment option for BD.
From clinical observations into the cells and genes
The use of the plant C. sativa dates back to several millennia, and from this use, it is known to possess several actions including inducing analgesia and euphoria, as well as serving as an anticonvulsant and inducing hallucinations (Walker & Huang, Reference Walker and Huang2002; Zuardi, Reference Zuardi2006; Atakan, Reference Atakan2012; Jones et al., Reference Jones, Glyn, Akiyama, Hill, Hill, Weston, Burnett, Yamasaki, Stephens, Whalley and Williams2012; Pearce et al., Reference Pearce, Mitsouras and Irizarry2014; Arjmand et al., Reference Arjmand, Vaziri, Behzadi, Abbassian, Stephens and Shabani2015). Many active ingredients have been extracted from C. sativa among which Δ9-tetrahydrocannabinol (Δ9-THC), cannabidiol (CBD), cannabigerol, cannabichromene, and cannabinol have drawn the greatest degree of attention as it is believed that these are the main components which confer exploitable pharmacological actions (Atakan, Reference Atakan2012; Andre et al., Reference Andre, Hausman and Guerriero2016). Prevalence of cannabis use/abuse has been reported in numerous studies to be high in patients with BD, and some of these reports have suggested that cannabis use may increase the risk of developing BD (Cassidy et al., Reference Cassidy, Ahearn and Carroll2001; Van Laar et al., Reference Van Laar, Van Dorsselaer, Monshouwer and De Graaf2007; Tijssen et al., Reference Tijssen, Van Os, Wittchen, Lieb, Beesdo and Wichers2010; Agrawal et al., Reference Agrawal, Nurnberger and Lynskey2011). In longitudinal studies, weekly to almost daily use of cannabis was associated with increased incidence of BD, whereas in adjusted models, only increased risk of (hypo)mania was noted (Feingold et al., Reference Feingold, Weiser, Rehm and Lev-Ran2015).
Age at onset
Cannabis has been suggested to reduce the age of onset of BD with psychotic features. In a study of 90 BD patients, not only was a high rate of cannabis use among these patients noted but also use of cannabis was associated with a decrease in the age of onset of BD symptoms which was more pronounced than the decrease in the age of onset of schizophrenia (De Hert et al., Reference De Hert, Wampers, Jendricko, Franic, Vidovic, De Vriendt, Sweers, Peuskens and van Winkel2011). These findings have led to the suggestion that there is a partly shared, pre-existing genetic liability for BD and schizophrenia; this liability could involve interactions with the ECS during neurodevelopment and, further, this liability could be unmasked upon cannabis exposure (De Hert et al., Reference De Hert, Wampers, Jendricko, Franic, Vidovic, De Vriendt, Sweers, Peuskens and van Winkel2011).
Although the aforementioned study was limited to BD patients with psychotic features, Lagerberg et al. Reference Lagerberg, Kvitland, Aminoff, Aas, Ringen, Andreassen and Melle(2014) conducted a large, representative clinical sample comprised of patients presenting with BD type 1 (BD-I), BD type 2 (BD-II), or BD not otherwise specified which concluded that a lower age of onset of BD is associated with cannabis use and does not rely on polydrug use and that BD occurrence is independent of the presence of either depressive or (hypo)manic mood or a history of psychosis (Lagerberg et al., Reference Lagerberg, Kvitland, Aminoff, Aas, Ringen, Andreassen and Melle2014). Further, the results of this study indicate that cannabis use may influence all subtypes of BD and that its use decreases age of onset of BD in a dose-dependent manner with a greater effect on depressive episodes (Lagerberg et al., Reference Lagerberg, Kvitland, Aminoff, Aas, Ringen, Andreassen and Melle2014). When taken together, the findings from both of the studies with large groups of BD patients showed that cannabis’ impact on BD is not gender specific which has also been previously reported (Öngür et al., Reference Öngür, Lin and Cohen2009; De Hert et al., Reference De Hert, Wampers, Jendricko, Franic, Vidovic, De Vriendt, Sweers, Peuskens and van Winkel2011; Lagerberg et al., Reference Lagerberg, Kvitland, Aminoff, Aas, Ringen, Andreassen and Melle2014).
In agreement with these findings, in another comprehensive study, cannabis use among BD patients (BD-I and BD-II) was associated with a significantly earlier age of onset of BD irrespective of the first manifestation of the disease, including whether the first episode was a state of depression or (hypo)manic (Lev-Ran et al., Reference Lev-Ran, Le Foll, McKenzie, George and Rehm2013). Likewise, it has been noted that cannabis use can dramatically affect the age of onset of both the first manic or depressive episodes about 5.6 and 5.9 years earlier, respectively, in diagnosed BD patients (Leite et al., Reference Leite, De Oliveira Nogueira, Do Nascimento, De Lima, Da Nóbrega, Da Silva Virgínio, Moreno, Sampaio, de Matos and Souza2015).
Onset of (hypo)manic or depressive episodes
Cannabis is likely to produce a range of psychological effects. There are many case studies reporting that cannabis use may induce the onset of clinical or subclinical symptoms of (hypo)mania (Henquet et al., Reference Henquet, Krabbendam, de Graaf, ten Have and van Os2006; Baethge et al., Reference Baethge, Hennen, Khalsa, Salvatore, Tohen and Baldessarini2008; Merikangas et al., Reference Merikangas, Herrell, Swendsen, Rössler, Ajdacic-Gross and Angst2008; Tijssen et al., Reference Tijssen, Van Os, Wittchen, Lieb, Beesdo and Wichers2010; Feingold et al., Reference Feingold, Weiser, Rehm and Lev-Ran2015; Mariangela Corbo, Reference Mariangela Corbo2015).
Recent findings of Tyler et al. Reference Tyler, Jones, Black, Carter and Barrowclough(2015) posited that cannabis use can be correlated with an increase in mania, positive affect, and depressive symptoms but not negative affect and reported that greater levels of positive effect were associated with an increase in the odds of cannabis use. Consistent with this study, when compared with individuals without co-occurring cannabis use, a significantly greater number of both (hypo)manic and depressive episodes with higher illness severity was seen in BD patients taking cannabis (Lev-Ran et al., Reference Lev-Ran, Le Foll, McKenzie, George and Rehm2013).
Many other studies, largely case studies, have also highlighted that there is an association between co-occurrence of cannabis use with exacerbation and even emergence of mania symptoms (Bertolín-Guillén et al., Reference Bertolín-Guillén, López-Arquero and Martínez-Franco2008; Khan & Akella, Reference Khan and Akella2009). In a systematic and meta-analysis review, Gibbs et al. Reference Gibbs, Winsper, Marwaha, Gilbert, Broome and Singh(2014) reported nearly a threefold increase in the advent of new manic episodes prior to the onset of disorder in cannabis users and a worsening of mania symptoms in cannabis users with pre-existing diagnosed BD.
Treatment outcome
Cannabis has been shown to contribute towards a reduction in treatment compliance among large samples of acutely manic BD patients who received anticonvulsants, antipsychotics, and/or lithium in a longitudinal study over the course of 1 year (van Rossum et al., Reference van Rossum, Boomsma, Tenback, Reed and van Os2009). Furthermore, cannabis users exhibited a more severe course of illness when compared with that of non-users (van Rossum et al., Reference van Rossum, Boomsma, Tenback, Reed and van Os2009). Two other studies also support these findings and suggested that cannabis users are likely to experience longer periods of mania than non-users and are non-compliant in utilisation of their medication during not only the acute phase but also maintenance, and hence, alternative therapeutic approaches might be required for this patient population (Baethge et al., Reference Baethge, Baldessarini, Khalsa, Hennen, Salvatore and Tohen2005; Gonzalez-Pinto et al., Reference Gonzalez-Pinto, Reed, Novick, Bertsch and Haro2010). Finally, poorer treatment outcomes and a higher frequency of developing rapid cycling and mixed episodes were reported in BD patients who presented with the comorbidity of cannabis use (Strakowski et al., Reference Strakowski, DelBello, Fleck, Adler, Anthenelli, Keck, Arnold and Amicone2007; Agrawal et al., Reference Agrawal, Nurnberger and Lynskey2011; Bally et al., Reference Bally, Zullino and Aubry2014).
Self-medication
There are several anecdotal reports and semi-structured, qualitative interviews which suggest that cannabis use acts as an antimanic and antidepressant that can alleviate the associated symptoms of both mania and depression, as well as reduce the side effects of lithium in BD patients, which implies that some use of cannabis in BD patients could reflect self-medication (Gruber et al., Reference Gruber, Pope and Brown1996; Grinspoon & Bakalar, Reference Grinspoon and Bakalar1998; Healey et al., Reference Healey, Peters, Kinderman, McCracken and Morriss2009). These findings are in accordance with the suggestion of Ashton et al. (Reference Ashton, Moore, Gallagher and Young2005) that both Δ9-THC and CBD have proved to be of great value in the management of anxiety, depression, and psychotic-like behaviours. Despite these observations, a human trial on two manic BD-I patients concluded that administration of oral CBD, even at tolerable high doses, did not show promising results for controlling manic episodes of BD (Zuardi et al., Reference Zuardi, Crippa, Dursun, Morais, Vilela, Sanches and Hallak2010; Micale et al., Reference Micale, Di Marzo, Sulcova, Wotjak and Drago2013).
A functional magnetic resonance imaging study
Hyperactivities of the right amygdala, left nucleus accumbens, and bilateral thalamus have been reported in non-cannabis using, adolescent bipolar individuals, whereas this over-activation is reduced in BD patients who are comorbid cannabis consumers. These interesting findings raised the question of whether cannabis use alters the functionality of brain areas involved in emotional processing and reward in BD subjects or whether differences are due to the presence of unique endophenotypes (Bitter et al., Reference Bitter, Adler, Eliassen, Weber, Welge, Burciaga, Shear, Strakowski and DelBello2014).
Into the genes
Relying on twin and family studies, it is evident that genetic factors contribute to the pathophysiology of BD (Kato, Reference Kato2007), and there are a large amount of studies being conducted to unravel the genetically-determined molecular signatures associated with BD, as well as to elucidate the mechanisms underlying varying responses to different treatment approaches (Rybakowski, Reference Rybakowski2013; Hou et al., Reference Hou, Heilbronner, Degenhardt, Adli, Akiyama, Akula, Ardau, Arias, Backlund, Banzato, Benabarre, Bengesser, Bhattacharjee, Biernacka, Birner, Brichant-Petitjean, Bui, Cervantes, Chen, Chen, Chillotti, Cichon, Clark, Colom, Cousins, Cruceanu, Czerski, Dantas, Dayer, Étain, Falkai, Forstner, Frisén, Fullerton, Gard, Garnham, Goes, Grof, Gruber, Hashimoto, Hauser, Herms, Hoffmann, Hofmann, Jamain, Jiménez, Kahn, Kassem, Kittel-Schneider, Kliwicki, König, Kusumi, Lackner, Laje, Landén, Lavebratt, Leboyer, Leckband, Jaramillo, MacQueen, Manchia, Martinsson, Mattheisen, McCarthy, McElroy, Mitjans, Mondimore, Monteleone, Nievergelt, Nöthen, Ösby, Ozaki, Perlis, Pfennig, Reich-Erkelenz, Rouleau, Schofield, Schubert, Schweizer, Seemüller, Severino, Shekhtman, Shilling, Shimoda, Simhandl, Slaney, Smoller, Squassina, Stamm, Stopkova, Tighe, Tortorella, Turecki, Volkert, Witt, Wright, Young, Zandi, Potash, DePaulo, Bauer, Reininghaus, Novák, Aubry, Maj, Baune, Mitchell, Vieta, Frye, Rybakowski, Kuo, Kato, Grigoroiu-Serbanescu, Reif, Del Zompo, Bellivier, Schalling, Wray, Kelsoe, Alda, Rietschel, McMahon and Schulze2016). In this regard, some studies examining BD have focused scrutiny on the role of genes encoding for players within the ECS. A recent study carried out on Turkish bipolar patients with the purpose of investigating CB receptor 1 gene (CNR1) single nucleotide polymorphisms (SNPs) reported that among three examined SNPs (rs6454674 T/G, rs806368 T/C, and rs1049353 A/G), only rs6454674 differed significantly in BD patients in comparison with healthy controls (Alpak et al., Reference Alpak, Copoglu, Geyik, Unal, Igci, Igci, Bozgeyik, Bulbul and Savas2014). This study also demonstrated that a significantly greater number of episodes of mania were associated with heterozygote rs6454674 polymorphisms, rather than homozygote ones, a relationship which was not observed for other clinical parameters including age at onset, duration of illness, and total number of BD episodes (Alpak et al., Reference Alpak, Copoglu, Geyik, Unal, Igci, Igci, Bozgeyik, Bulbul and Savas2014).
Genetic associations between BD, pharmacological management of BD, and the CB2 have also been examined. In an Italian cohort, the presence of the CB2 gene (CNR2) polymorphism, rs41311993 (524C/A), was significantly associated with BD, without any significant association in the SNPs of rs2229572 (1073C/T) or rs2501432 (315A/G) noted (Minocci et al., Reference Minocci, Massei, Martino, Milianti, Piz, Di Bello, Sbrana, Martinotti, Rossi and Nieri2011). This study unfortunately did not include pharmacogenetic evaluation. Although the sample sizes were small and therefore need to be corroborated in larger patient groups, when taken together, these reports are suggestive of a role for both CB receptors in BD and leave the door open to considerations that different genetic polymorphisms could confer varying responses to different treatment strategies. Countering this suggestion, Pisanu et al. Reference Pisanu, Congiu, Costa, Sestu, Chillotti, Ardau, Deiana, Manchia, Squassina and Del Zompo(2013) have reported that there are no significant associations with polymorphisms in BD patients which could substantiate the involvement of SNPs of CNR1. Further, in the same study, SNPs of fatty acid amide hydrolase (FAAH) or N-acyl phosphatidyl ethanolamine phospholipase D, which are two of the major enzymes responsible for endogenous CB inactivation and biosynthesis, respectively, were also not found to be associated with BD. Additionally, none of the SNPs of players in the ECS which were examined showed an association with responses to treatment with lithium (Pisanu et al., Reference Pisanu, Congiu, Costa, Sestu, Chillotti, Ardau, Deiana, Manchia, Squassina and Del Zompo2013). Along the same lines, Monteleone et al. Reference Monteleone, Bifulco, Maina, Tortorella, Gazzerro, Proto, Di Filippo, Monteleone, Canestrelli, Buonerba, Bogetto and Maj(2010) failed to show that the CNR1 SNP, rs1049353 (1359 G/A), was associated with BD in a caucasian population; however, a trend was noted when the association of the FAAH SNP, rs324420, was examined. Further, no differences in the expression of the CNR1 and CNR2 genes were seen postmortem in the prefrontal cortex of BD patients compared with aged-matched controls (Choi et al., Reference Choi, Le, McGuire, Xing, Zhang, Li, Parker, Johnson and Ursano2012). Moreover, immunohistochemistry analyses of postmortem brain tissue of bipolar patients revealed no significant changes in thedensity of CB1 receptors in the anterior cingulate cortex. However, a marked decrease in numerical density of CB1-immunoreactive glial cells following administration of first-generation antipsychotic drugs was seen (Koethe et al., Reference Koethe, Llenos, Dulay, Hoyer, Torrey, Leweke and Weis2007; Leweke & Koethe, Reference Leweke and Koethe2008).
Into the cells: inflammation, the arachidonic acid pathway, endocannabinoids, and BD
As investigations of the pathophysiology underlying BD have increased, an idea has emerged that BD might represent an inflammatory disorder, which could lead to the consideration that BD is a disorder not only of the brain but also of the body (Leboyer et al., Reference Leboyer, Soreca, Scott, Frye, Henry, Tamouza and Kupfer2012). A potential role for inflammation in the etiology of BD is based partly on the findings that pro-inflammatory cytokines, such as interleukin-1 (IL-1), IL-2, IL-4, IL-6, and tumour necrosis factor-alpha (TNF-α), are present at elevated levels when mania is dominant in BD patients, and IL-6 is explicitly increased during the depression phase. Elevated levels of all of these pro-inflammatory cytokines, with the exception of IL-4 return back to normal levels when bipolar individuals become euthymic (O’Brien et al., Reference O’Brien, Scully, Scott and Dinan2006; Kim et al., Reference Kim, Jung, Myint, Kim and Park2007; Ortiz-Domínguez et al., Reference Ortiz-Domínguez, Hernández, Berlanga, Gutiérrez-Mora, Moreno, Heinze and Pavón2007; Brietzke et al., Reference Brietzke, Kauer-Sant’Anna, Teixeira and Kapczinski2009a,b; Hamdani et al., Reference Hamdani, Tamouza and Leboyer2012).
Differences in the levels of some of the ILs appear to vary at different stages of BD. In the early stages of BD, IL-10 is elevated, whereas TNF-α and IL-6 are at high levels at both early and late phases of BD (Berk et al., Reference Berk, Kapczinski, Andreazza, Dean, Giorlando, Maes, Yücel, Gama, Dodd, Dean, Magalhães, Amminger, McGorry and Malhi2011; Leboyer et al., Reference Leboyer, Soreca, Scott, Frye, Henry, Tamouza and Kupfer2012). Moreover, treatment with mood stabilisers has been shown to return the higher levels of pro-inflammatory cytokines back to their baseline levels (Boufidou et al., Reference Boufidou, Nikolaou, Alevizos, Liappas and Christodoulou2004; Hamdani et al., Reference Hamdani, Tamouza and Leboyer2012). Presence of heightened levels of C-reactive protein has been associated with both the manic and depressive states, as well as with the severity of manic symptoms (Wadee et al., Reference Wadee, Kuschke, Wood, Berk, Ichim and Maes2002; Dickerson et al., Reference Dickerson, Stallings, Origoni, Boronow and Yolken2007; Huang & Lin, Reference Huang and Lin2007; Cunha et al., Reference Cunha, Andreazza, Gomes, Frey, Da Silveira, Gonçalves and Kapczinski2008; De Berardis et al., Reference De Berardis, Conti, Campanella, Carano, Scali, Valchera, Serroni, Pizzorno, D’Albenzio, Fulcheri, Gambi, La Rovere, Cotellessa, Salerno and Ferro2008). Interestingly, BD and players in inflammation processes may be associated at the molecular level as they share genetic polymorphisms and gene expression (Goldstein et al., Reference Goldstein, Kemp, Soczynska and McIntyre2009).
Another connection between BD and inflammation is a link detected between BD and activation of microglia, a phenomenon that can amplify both pro- and anti-inflammatory cytokines (Ekdahl, Reference Ekdahl2012; Weitz & Town, Reference Weitz and Town2012; Stertz et al., Reference Stertz, Magalhães and Kapczinski2013). CB2 receptors that are found in the periphery are mostly located within the immune system, which suggests that CB2 receptors and the ECS play a role in regulating immune cell functions (Arjmand et al., Reference Arjmand, Vaziri, Behzadi, Abbassian, Stephens and Shabani2015; Turcotte et al., Reference Turcotte, Blanchet, Laviolette and Flamand2016). Consistent with this, studies on CB2 receptor knockout mice, in which the CNR2 gene has been inactivated, have confirmed the crucial role for CB2 receptor as an immunomodulator and extended the notion that CB2-selective agonists may well improve inflammation and act as immunosuppressive (Ashton & Glass, Reference Ashton and Glass2007; Turcotte et al., Reference Turcotte, Blanchet, Laviolette and Flamand2016). Additionally, Ehrhart et al. Reference Ehrhart, Obregon, Mori, Hou, Sun, Bai, Klein, Fernandez, Tan and Shytle(2005) have provided mechanistic insights regarding how a CB2-selective agonist attenuates release of microglial, pro-inflammatory cytokines and suppresses microglial activation, which is interesting in light of the heightened microglial activation seen in BD. CB2 agonists have been shown to abrogate the activity of the immune system by affecting several pathways (Malfitano et al., Reference Malfitano, Basu, Maresz and Bifulco2014). Stimulation of CB2 receptor, which is coupled to Gi protein, dampens the activity of adenylyl cyclase resulting in diminished cyclic adenosine monophosphate (cAMP) and in a subsequent reduction in the activity of protein kinase A that is responsible for phosphorylation of cAMP response element binding protein (CREB) (Malfitano et al., Reference Malfitano, Basu, Maresz and Bifulco2014). CREB is a transcription factor in charge of modulating both proliferation and differentiation of the immune system’s components (Malfitano et al., Reference Malfitano, Basu, Maresz and Bifulco2014). In addition, activation of CB2 receptor can affect several cell survival pathways, such as MAPK, ERK, STAT1, and JAK (Ehrhart et al., Reference Ehrhart, Obregon, Mori, Hou, Sun, Bai, Klein, Fernandez, Tan and Shytle2005; Malfitano et al., Reference Malfitano, Basu, Maresz and Bifulco2014). CB2 agonists have demonstrated a capability to hamper interferon gamma, a key element in processes leading to suppression of expression of CD40, microglial TNF-α, production of nitric oxide, and STAT1/JAK phosphorylation with a net result of immune system inhibition (Ehrhart et al., Reference Ehrhart, Obregon, Mori, Hou, Sun, Bai, Klein, Fernandez, Tan and Shytle2005).
While actions at the CB2 seem to inhibit inflammatory processes, stimulation of the CB1 has been shown to lead to activation of inflammatory mediators. The endogenous CBs, anandamide, and 2-arachidonoylglycerol are substrates for cyclooxygenase-2 (COX-2) and via oxygenation are converted to prostaglandin glyceryl esters, prostaglandin ethanolamides and arachidonic acid (AA)-derived prostaglandin E2 (PGE2), which results in a reduction in the amount of endocannabinoids (Yang et al., Reference Yang, Zhang, Andreasson and Chen2008; Turcotte et al., Reference Turcotte, Chouinard, Lefebvre and Flamand2015). Δ8-THC, a more chemically stable isoform of Δ9-THC, as well as Δ9-THC, and a potent CB1 agonist, HU-210, were found to augment the amount of PGE2 through actions at the CB1 receptors, which could be antagonised by COX-2 inhibitors (Yamaguchi et al., Reference Yamaguchi, Shoyama, Watanabe and Yamamoto2001; Kim et al., Reference Kim, Rapoport and Rao2011). The effects of CB1 on AA production are interesting in light of findings from postmortem investigations of the brains of patients with BD, which revealed an up-regulation of the AA cascade (Kim et al., Reference Kim, Rapoport and Rao2011).
Mood stabilisers such as lithium, carbamazepine, and lamotrigine have been shown to downgrade AA turnover and thus diminish PGE2 concentration specifically by lowering the expression of COX-2, whereas valproate tends to affect both COX-1 and COX-2 (Sublette et al., Reference Sublette, Russ and Smith2004; Rapoport, Reference Rapoport2014). Although two rather old studies are indicative that use of some non-steroidal anti-inflammatory drugs (NSAIDs) such as indomethacin and tolmetin may exacerbate the associated symptoms of mania, this could be due to their non-selective inhibitory action on both COX-1 and COX-2. However, there is also a case report that COX-2 selective NSAIDs induced hypomania despite maintenance of treatment with mood-enhancing drugs, and symptoms remitted following 3 days of discontinuation of the NSAID (Sotsky & Tossell, Reference Sotsky and Tossell1984; Bishop et al., Reference Bishop, Bisset and Benson1987; Mahajan et al., Reference Mahajan, Mahajan and Mittal2012).
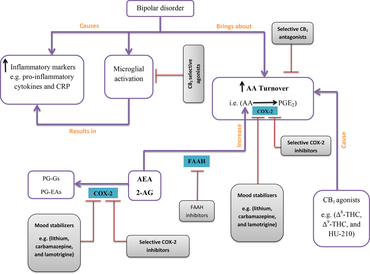
When taken together, it is plausible that enhancing endocannabinoid level by utilising selective COX-2 inhibitors or endocannabinoid hydrolysis inhibitors in combination with CB1-selective antagonists and CB2-selective agonists represents a new strategy to not only manage BD in treatment-resistant patients but also vigorously dissect the exact underlying molecular pathways and potentially reveal further improved therapeutic opportunities to better manage BD (Fig. 1).

Fig. 1. Bipolar disorder (BD) is associated with some changes in the arachidonic acid (AA) cascade and inflammatory markers. This concept map presents a simplified schematic of changes in the AA cascade and inflammatory markers in the course of BD as well as providing a concise proposal on how to modulate these alterations. In BD, increased AA turnover and increased level of both pro-inflammatory cytokines and C-reactive protein (CRP) have been detected. Activation of microglial cells itself can induce an increase in the concentration of inflammatory markers which can be inhibited by use of cannabinoid receptor type 2 (CB2)-selective agonists (red lines are indicative of inhibitory mode of action). In another pathway, cyclooxygenase-2 (COX-2) oxygenates AA to classic prostaglandin E2 (PGE2), whereas anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are converted to new PGs called prostaglandin glyceryl esters (PG-Gs) and prostaglandin ethanolamides (PG-EAs). Preventing AA metabolism and enhancing the amount of endocannabinoids might also lead to the assuagement of BD symptoms, a strategy which can be achieved by using CB1-selective antagonists, mood stabilisers, and selective COX-2 inhibitors.
Rimonabant: a controversial scenario?
It has been well proven that rimonabant, an inverse agonist of CB1 receptors, can induce depression and anxiety, whereas the opposite anxiolytic and antidepressant effects of drugs that boost CB1 receptor activity have been established (Moreira & Crippa, Reference Moreira and Crippa2009; Kruk-Slomka et al., Reference Kruk-Slomka, Michalak and Biala2015). Moreover, acute pretreatment with a CB1 antagonist, AM251, has been demonstrated to abolish the antidepressive effects of desipramine (Hill & Gorzalka, Reference Hill and Gorzalka2005). Such observations were followed by genetic studies on CB knockout mice that exhibited a depression-like phenotype which resembled that triggered in mice who underwent mild chronic stresses (Beyer et al., Reference Beyer, Dwyer, Piesla, Platt, Shen, Rahman, Chan, Manners, Samad, Kennedy, Bingham and Whiteside2010). The behavioural profile of the knockout mice supported the suggestion that CB1-deficient mice can be used as an animal model for depression (Valverde & Torrens, Reference Valverde and Torrens2012). There are also studies of rimonabant that are indicative of either no effect on depression or anxiety or an antidepressant-like effect which makes the evaluation of the role played by this drug in depression difficult (Gobbi et al., Reference Gobbi, Bambico, Mangieri, Bortolato, Campolongo, Solinas, Cassano, Morgese, Debonnel, Duranti, Tontini, Tarzia, Mor, Trezza, Goldberg, Cuomo and Piomelli2005; Griebel et al., Reference Griebel, Stemmelin and Scatton2005; Adamczyk et al., Reference Adamczyk, Gołda, McCreary, Filip and Przegaliński2008; Steiner et al., Reference Steiner, Marsicano, Nestler, Holsboer, Lutz and Wotjak2008). However, we have suggested that CB1 antagonists may modulate mood by suppressing AA turnover and that they might act as a mood stabiliser. It should be noted that a preferable mood stabiliser should reduce mood swings and maintain euthymia, as well as prevent episodic relapse of the illness (Malhi et al., Reference Malhi, Porter, Irwin, Hamilton, Morris, Bassett, Baune, Boyce, Hopwood, Mulder, Parker, Mannie, Outhred, Das and Singh2018). It has been previously demonstrated that mood stabilisers are capable of targeting brain’s AA signaling and can stabilise mood by downregulation of AA cascade (Chang et al., Reference Chang, Contreras, Rosenberger, Rintala, Bell and Rapoport2001; Rao & Rapoport, Reference Rao and Rapoport2009). Here, we suggest a mood stabilising mode of action for CB1 antagonists and not an antidepressant effect. Moreover, rimonabant is an inverse agonist, and development of a putative neutral antagonist may diminish the depression-like effect of such agents (Giraldo, Reference Giraldo2010; Ward & Raffa, Reference Ward and Raffa2011). However, at this time, this remains a speculation, and the potential for CB1 antagonists to stabilise mood needs to be rigorously examined.
Conclusion
Although data from the studies are somewhat inconsistent and no direct measurement of the plasma levels of endocannabinoids and their associated enzymes has yet been reported in BD, it is apparent that the ECS is involved in control of mood. Different routes of administration, rather small sample sizes, a large variety of active ingredients of cannabis, and different doses may affect the results of clinical studies and make the final conclusion obscure and controversial. The first evidence of this role sources from clinical observations, which are largely anecdotal, that triggered experimental studies which have shown that BD and endocannabinoids may share a link (Table 1). Cannabis has been shown to affect the age of onset of BD, severity, and the number of affective episodes. It has also been demonstrated that both AA and inflammatory pathways can play a part in the pathophysiology of BD, and a link from the ECS to inflammatory pathways has been strongly established. After consideration of these studies, the majority of which have been molecular, we proposed that COX-2 inhibitors, endocannabinoid hydrolysis inhibitors, CB1-selective antagonists, and CB2-selective agonists may lead to remarkable advances pertaining to pharmacotherapy of BD based on modulation of the ECS, and this approach offers a brand-new treatment strategy to broaden the arsenal available to pharmacologically mange BD. Since increased turnover of AA is evident in BD and disparate classes of currently available mood stabilisers share the mechanism of diminishing AA turnover, activation of CB2 receptors might offer BD patients’ stabilisation of mood with the same final outcome. The lipophilic nature of the CB ligands, as well as their long biological half-life, bequeaths them the advantage of crossing the blood–brain barrier easily and could possibly reduce the risk of overdose (Stratton et al., Reference Stratton, Allen, Wu and Shafer2013) in BD patients, some of whom are predisposed to suicidal ideation and attempts. Given the failure of control of BD in a subset of patients with the medications currently available, when taken together with the studies examining a role of endocannabinoids in control of mood, examination is warranted of whether selective activation and inhibition of endocannabinoid receptors can serve as a suitable treatment approach for BD.
Table 1. Endocannabinoids and BD; the story so far
BD, bipolar disorder; CNR1, cannabinoid receptor 1; CNR2, cannabinoid receptor 2; AA, arachidonic acid; PGE2, prostaglandin E2; COX-2, cyclooxygenase-2.
Author ORCIDs
Mohammad Shabani 0000-0002-2082-5849
Author contributions
SA has conceived and designed the concept and road map of the study, searched the literature, and drafted the manuscript. She also designed the concept map and box. MB has searched the literature, categorised the searched papers, and helped design the study and box. KAK has critically reviewed the manuscript for its content, originality, usage of English language, and accuracy of the interpreted data. SM and AS have reviewed the manuscript for the intellectual content and approved the final version for submission. MS has critically reviewed the manuscript, designed the study, and helped in manuscript preparation. He is the archival author and attests to the integrity of the original data and the analysis reported in this manuscript. All authors have made substantive contribution and attest to approving the final manuscript.
Conflict of interest
The authors declare that no competing and financial interests exist.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution.