Book contents
- Frontmatter
- Contents
- Preface
- Notation
- 1 Introduction
- 2 Temperature, heat, work, energy, and enthalpy
- 3 The second law of thermodynamics: the entropy function
- 4 Gibbs and Helmholtz energy functions and open systems
- 5 Conditions of equilibrium and stability: the phase rule
- 6 Partial molar quantities
- 7 Ideal gases and real gases
- 8 Liquids and solids: reference and standard states
- 9 Thermochemistry
- 10 Phase equilibrium
- 11 Chemical equilibrium
- 12 Equilibria in electrochemical systems
- 13 Surface effects
- 14 Equilibrium conditions in the presence of an external field
- 15 The third law of thermodynamics
- Appendices
- A SI units and fundamental constants
- B Molar heat capacities at constant pressure for selected substances
- C Thermodynamic data
- D Standard reduction potentials in aqueous solutions
- Cited references and selected bibliography
- Subject index
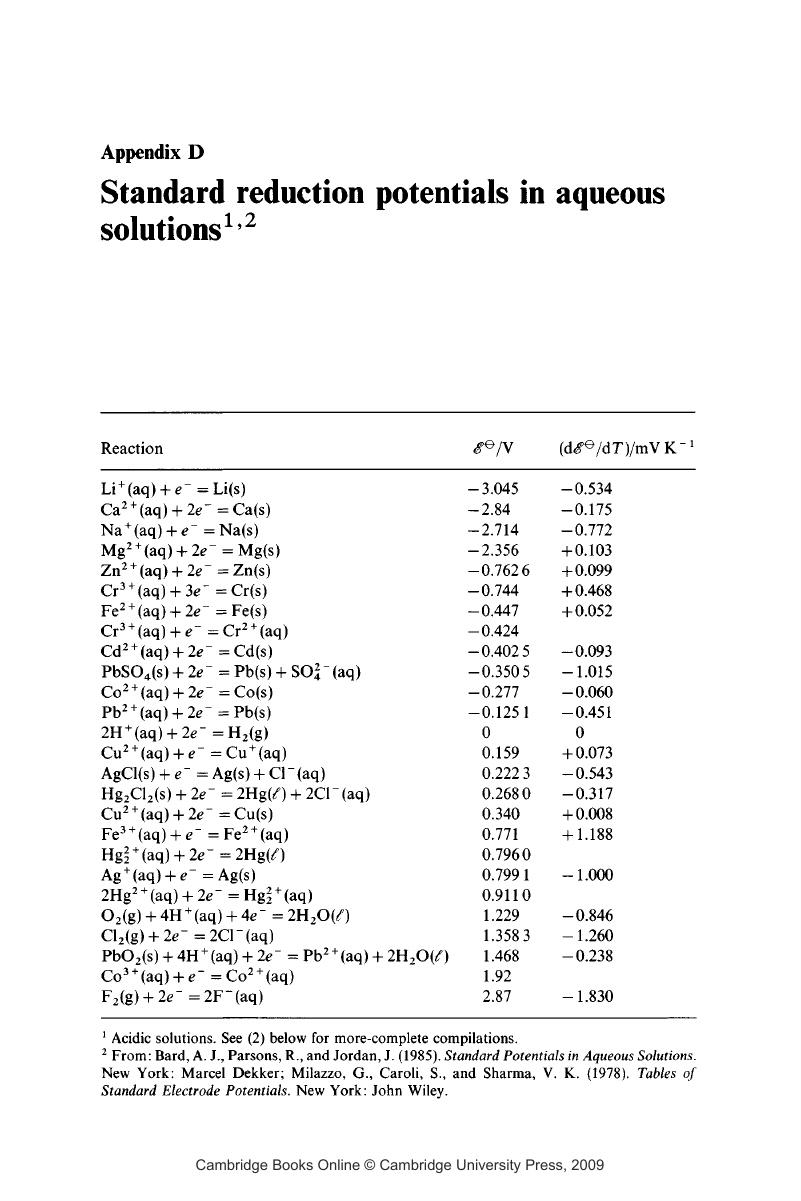
D - Standard reduction potentials in aqueous solutions
Published online by Cambridge University Press: 28 October 2009
- Frontmatter
- Contents
- Preface
- Notation
- 1 Introduction
- 2 Temperature, heat, work, energy, and enthalpy
- 3 The second law of thermodynamics: the entropy function
- 4 Gibbs and Helmholtz energy functions and open systems
- 5 Conditions of equilibrium and stability: the phase rule
- 6 Partial molar quantities
- 7 Ideal gases and real gases
- 8 Liquids and solids: reference and standard states
- 9 Thermochemistry
- 10 Phase equilibrium
- 11 Chemical equilibrium
- 12 Equilibria in electrochemical systems
- 13 Surface effects
- 14 Equilibrium conditions in the presence of an external field
- 15 The third law of thermodynamics
- Appendices
- A SI units and fundamental constants
- B Molar heat capacities at constant pressure for selected substances
- C Thermodynamic data
- D Standard reduction potentials in aqueous solutions
- Cited references and selected bibliography
- Subject index
Summary
- Type
- Chapter
- Information
- Thermodynamics of Chemical Systems , pp. 422Publisher: Cambridge University PressPrint publication year: 1990

