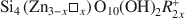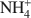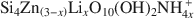Smectites are one of the most important groups of phyllosilicates found in soils and sediments, and certainly one of the most difficult to study. New information about the formation mechanisms, impact of structural features on surface properties, and long-term stability of smectites can best be gained from the systematic study of single-phase specimens. In most instances, these specimens can only be obtained through synthesis under controlled conditions. Syntheses of smectites have been attempted (1) at ambient pressure and low-temperature (<100°C), (2) under moderate hydrothermal conditions (100–1000°C, pressures to several kbars), (3) under extreme hydrothermal conditions (>1000°C or pressures >10 kbars), and (4) in the presence of fluoride. Of these approaches, syntheses performed under moderate hydrothermal conditions are the most numerous and the most successful in terms of smectite yield and phase-purity. Using hydrothermal techniques, high phase-purity can be obtained for beidellites and several transition-metal smectites. However, synthesis of montmorillonite in high purity remains difficult. Starting materials for hydrothermal syntheses include gels, glasses, and other aluminosilicate minerals. The presence of Mg2+ seems to be essential for the formation of smectites, even for phases such as montmorillonite which contain low amounts of Mg. Highly crystalline smectites can be obtained when extreme temperatures or pressures are used, but other crystalline impurities are always present. Although the correlation between synthesis stability fields and thermodynamic stability fields is good in many instances, metastable phases are often formed. Few studies, however, include the additional experiments (approach from under-and over-saturation, reversal experiments) needed to ascertain the conditions for formation of thermody-namically stable phases. Thorough characterization of synthetic products by modern instrumental and molecular-scale techniques is also needed to better understand the processes leading to smectite formation.