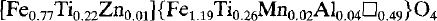Gabbroic saprolite at Mont Mégantic, Quebec, was studied in detail mineralogically to gain a better understanding of the origin of ferruginous smectite reported previously from these rocks. The parent rock is a ferrogabbro composed of plagioclase, augite, calc-alkalic amphiboles, biotite, olivine, magnetite, ilmenite, and apatite. Extensive weathering has decomposed most of the mafic minerals and magnetite to goethite, lepidocrocite, and iron-rich clay minerals, which occur in numerous microcracks distributed irregularly in the outer shells of boulders and in cracks and fissures in the bedrock. Some of the felsic minerals have altered to kaolinite. The secondary minerals and the more resistant primary minerals, such as plagioclase, ilmenite, and apatite, have subsequently moved to the lower part of the saprolite. The major ferruginous clay minerals present are smectite and vermiculite, which are compositionally similar, except that the smectite is slightly richer in SiO2 and MgO and poorer in Fe2O3 than the vermiculite.
To establish a structural formula for the ferruginous smectite, the oxidation state of Fe in a sample treated with dithionite-citrate-bicarbonate was examined by Mössbauer spectroscopy. All structural iron was found to be ferric. The calculated structural formula of a Na-saturated sample is:$$N{a_{0.61}}\left( {M{g_{1.39}} + Fe_{0.85}^{3 + }A{l_{0.17}}M{n_{0.03}}} \right)\left( {S{i_{3.49}}A{l_{0.51}}} \right){O_{10}}{\left( {OH} \right)_2},$$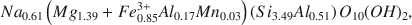
which has a total octahedral population of 2.44. Octahedral (Mg + Mn) exceeds octahedral (Fe3+ + Al). The 060 reflection at 1.527 Å is closer to the 1.530-Å value typical of saponite than to the 1.518-Å value typical of nontronite. The infrared spectra of the ferruginous smectite is also similar to that of saponite. Thus, the mineral is best described as a ferrian saponite. Ferroan saponite originally formed, and due to subsequent oxidation, some Fe3+ was expelled from the octahedral sheets, giving rise to a ferrian saponite containing octahedral vacancies. The expelled iron presumably formed the iron oxyhydroxides that coexist with the saponite.

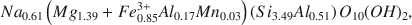
 exchange at low-
exchange at low-