Introduction
In the USA, 15% of diabetics develop chronic wounds in their foot, of which 14–43% result in amputations due to their non-healing status.[Reference Sheahan, Hamdan, Veraldi, McArthur, Skillman, Campbell, Scovell, LoGerfo and Pomposelli1] The main treatments for chronic diabetic foot ulcers include hydrogel/polymer-based wound dressings, negative pressure therapy, and skin grafts.[Reference Yazdanpanah, Nasiri and Adarvishi2, Reference Han and Ceilley3] Adequate issue oxygenation plays an important role in all stages of wound healing (inflammation to tissue remodeling). Many chronic wounds, including diabetic foot ulcers, have hypoxic regions that prevent, impede, and delay the healing process. Several oxygen-based therapies have been proposed and deployed for chronic wound treatment. These include hyperbaric oxygen (HBO) therapy, topical oxygen (TO) therapy, and continuous diffusion of oxygen (CDO).[Reference Howard, Asmis, Evans and Mustoe4–Reference Goldman8] In HBO therapy, the patient is placed in a HBO chamber (1.4–3.0 atm) for 90 min per day, 4 or 5 days per week. In addition to reducing wound hypoxia, HBO has been reported to promote fibroblast proliferation, increase collagen production, enhance immune function, and stimulate angiogenesis. This technique, however, is cumbersome, requiring sophisticated/bulky equipment (hyperbaric chamber and a large oxygen source) and trained personnel. In addition, it can result in systemic side effects such as myopia, pneumothorax, and hyperoxia-induced toxicity. An alternative but similar approach is TO therapy; this technique creates a sealed and localized oxygen-rich environment around the limb or affected area with barometric pressure slightly above atmospheric pressure while providing oxygen at a flow rate in the range of 5–60 L/min.[Reference Howard, Asmis, Evans and Mustoe4, Reference Dissemond, Kröger, Storck, Risse and Engels9] TO therapy improves the oxygen delivery efficiency by releasing oxygen directly at the hypoxic portion of the wound. TO offers several other advantages, including reduced risk of oxygen toxicity (due to a reduction in the exposed tissue), increased portability, and lower cost; enabling its use in a more diverse set of environments such as homes and retail clinics. However, their high flow rates may cause tissue desiccation, dramatically reducing the oxygen solubility and transportation into the tissue unless a counteracting humidification system is used. Additionally, TO therapy still requires an external oxygen source, which often prevents or limits patient mobility during treatment.
CDO therapy is a recent development aimed at mitigating the shortcomings associated with the hyperbaric and TO therapies. CDO delivers continuous oxygen to an occluded, moist wound site at much lower flow rates of 3–12 mL/h for 24 h 7 days a week for up to several weeks or months, depending on the wound status. Currently, there are several embodiments of CDO technology in the market. These include EPIFLO (Ogenix Inc.) and NATROX (InotecAMD Ltd.). Both systems are able to concentrate oxygen from the ambient air to nearly 100% and pump it through a soft tubing into the wound dressing at the rates of 3–13 mL/h for up to 15 days (at atmospheric pressure).[Reference Dissemond, Kröger, Storck, Risse and Engels9–Reference Curran, Fisher, Hayes, Loftus and Sequeira13] Another product, TransCu O2 (EO2 Concepts Inc.), utilizes a similar mechanism to generate atmospheric oxygen but controls its delivery rate within a range of 3–10 mL/h through embedded feedback electronics, providing a more flexible and controllable oxygen therapy. All these products allow for patient mobility and limit oxygen exposure to only the wound bed. However, such convenience comes at a cost. The price of these systems is in the range of $1000 per small wound or $70–100 per day,[Reference Banks and Ho12] making them unavailable to many affected individuals (50–79% of Americans with diabetic ulcers are unemployed, have retired early, or are unable to work,[Reference Goodridge, Trepman and Embil14] resulting in about 5 million Americans with diabetic ulcers who live at or below the poverty threshold[15]). A more socially and economically impactful solution would be one that is more affordable, easy to use, and provides localized oxygen generation that complements the heterogeneous nature of chronic wounds.
As a low-cost alternative to CDO and specifically for diabetic foot ulcers, we have developed a silicone-based insole with selective laser-ablated regions to deliver oxygen to wound regions on the sole of the foot. Although polydimethylsiloxane (PDMS) on its own cannot absorb excess exudate from deep wounds, the insole can be worn over a standard exudate-absorbing dressing such as a polyurethane-based commercial wound dressing foam, which still allows oxygen permeation (albeit after a time delay).[Reference Moura, Dias, Carvalho and De Sousa16, Reference Khil, Il Cha, Kim, Kim and Bhattarai17] Compared with most other polymers, silicone (specifically PDMS) has a higher oxygen permeability, which can be further chemically improved by decreasing the crosslinking ratio or increasing its porosity. Such chemical-level control, however, is not convenient for mass production since it would require selective heterogeneous chemical composition throughout a single substrate to achieve flux-based control of oxygen delivery. As a manufacturable alternative, we introduce a CO2 laser machining technique for PDMS surface engineering to reduce the thickness and increase the surface-to-volume ratio of the PDMS membrane, thus enhancing the oxygen permeability in the targeted regions. The technique enables creation of precise and customized oxygen generating regions that match the hypoxic wound profile. Direct micro-patterning with a CO2 laser is advantageous for mass customization due to its ease of pattern definition, low processing cost, rapid production speed, and high scalability for manufacturing at different production levels.[Reference Klank, Kutter and Geschke18]
Working principle
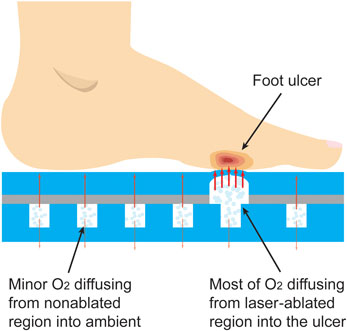
The working mechanism of the oxygen-releasing insole is demonstrated in Fig. 1. The insole consists of two PDMS layers, a thick one at the bottom and a thin one on the top. The former features an array of 1 cm-diameter pillars distributed with a spatial percentage ratio of 25% (mm3/mm3), which is designed to offer a balance between mechanical support (pillars) and stored oxygen (pillar-free space). The pillar arrangement also provides a means for tuning the mechanical strength of this insole if needed in future designs. The top PDMS layer is laser-machined at one or more regions with a predetermined or custom pattern. The two layers are bonded together and oxygen is loaded in the insole during or after fabrication. The insole is stored in an air-tight package until ready for use. When inserted in the shoe, oxygen is released once pressure is applied by either standing or walking. The pressure causes oxygen to permeate the thin membrane primarily through the laser-ablated regions, directly into the wound area. Without applied pressure, the insole will continue to release oxygen, but at a lower rate.
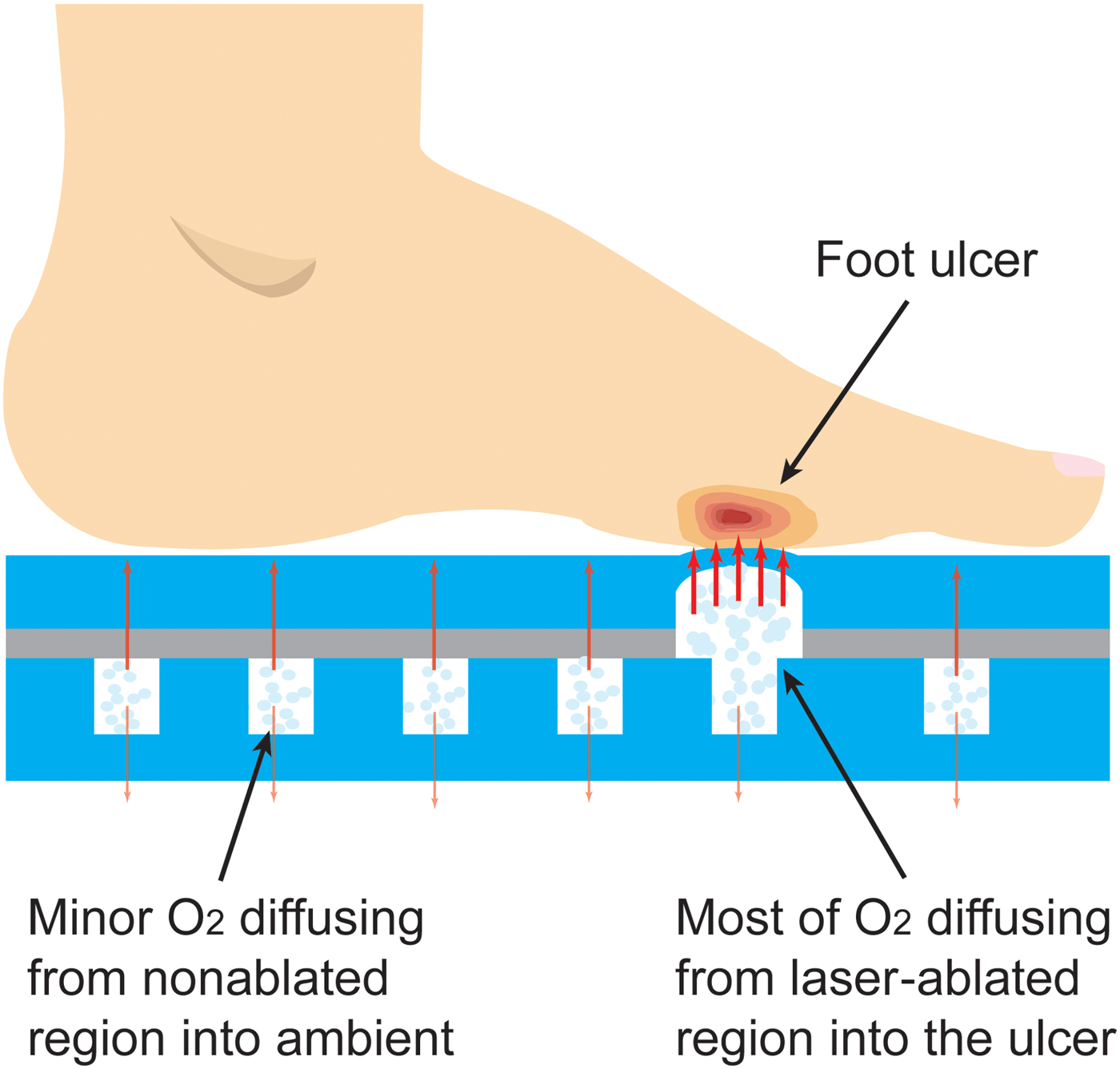
Figure 1. Conceptual illustration of the insole working mechanism. At high pressures applied by either standing or walking oxygen permeates the thin membrane primarily through the laser-etched regions directly into the wound area; at normobaric pressure, the insole continues to release oxygen at a lower rate.
Experimental
Fabrication
The fabrication of the insole is shown in Figs. 2(a)–2(c). For the top layer of the insole, first, a thin layer of PDMS, 1 mm-thick, is bonded (via oxygen plasma) to one side of a transparent adhesive transfer tape (3M 300LSE). A selective area in which the oxygen has to be transported to the foot is then laser-ablated to certain thickness. Next, the bottom thicker layer is fabricated using a 4 mm-thick PDMS. This layer is cast and cured in a 3D printed mold at 70 °C for 3 h and then bonded (via oxygen plasma) to the other side of the adhesive transfer tape holding the top layer. The assembled insole is pressed by a weight and annealed at 70 °C for 2 h to increase the mechanical strength. Finally, the insole is filled with pure oxygen through a syringe pump and stored inside an oxygen-rich sealed plastic bag until ready for use.
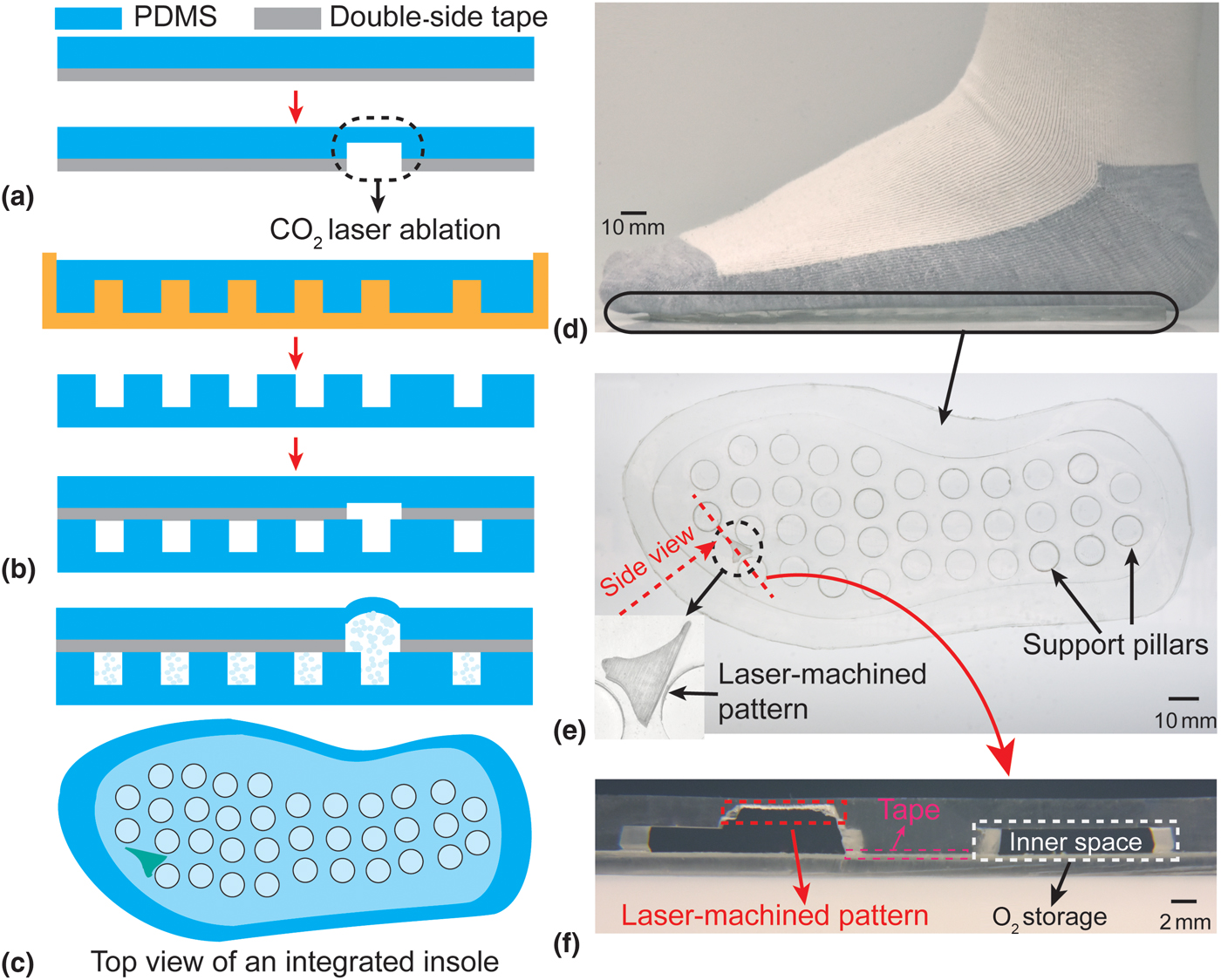
Figure 2. (a)–(c) Fabrication process of the insole; (d and e) photographs of the fabricated insole (inset: the magnified top view of laser-ablated pattern); and (f) the magnified side view of the laser-ablated pattern, surrounded by the non-ablated regions serving as the inner space of the insole for the oxygen storage. Note that laser-machining can be applied on either inner side of the insole, e.g., thicker layer is laser-ablated in (f); once the desired thickness of the PDMS is achieved, the working mechanism and functionality of the insole should be maintained.
Characterizations
The insoles were characterized in terms of the mechanical strength. These included the measurement of both peel strength and bond strength for various types of bonding (i.e., with and without an intermediate bonding agent).[Reference Eddings, Johnson and Gale19] The peel strength was measured by using a universal test machine (ADMET) to peel (20 × 50 mm2) strips at a peel rate of 1 mm/s using three different bonding methods [Fig. 3(a)]. The bond strength was measured using a pressurized test chamber (an 80 mm diameter and 6.1 mm height PDMS cylinder with a 1 cm diameter and 1.8 mm height inner chamber). With the chamber connected to a pressure gage (DPG4000, OMEGA Engineering Inc.) and a syringe pump, DI water was pumped into the chamber at a rate of 1 mL/min and the pressure was continuously monitored [Fig. 3(b)]. The maximum bond strength was defined as the recorded pressure immediately before device failure. A set of three samples were evaluated in both scenarios for each bonding method. As a practical demonstration of robustness, the long-time reliability of the insole was also tested by wearing it for one day and subsequently inspecting it visually.
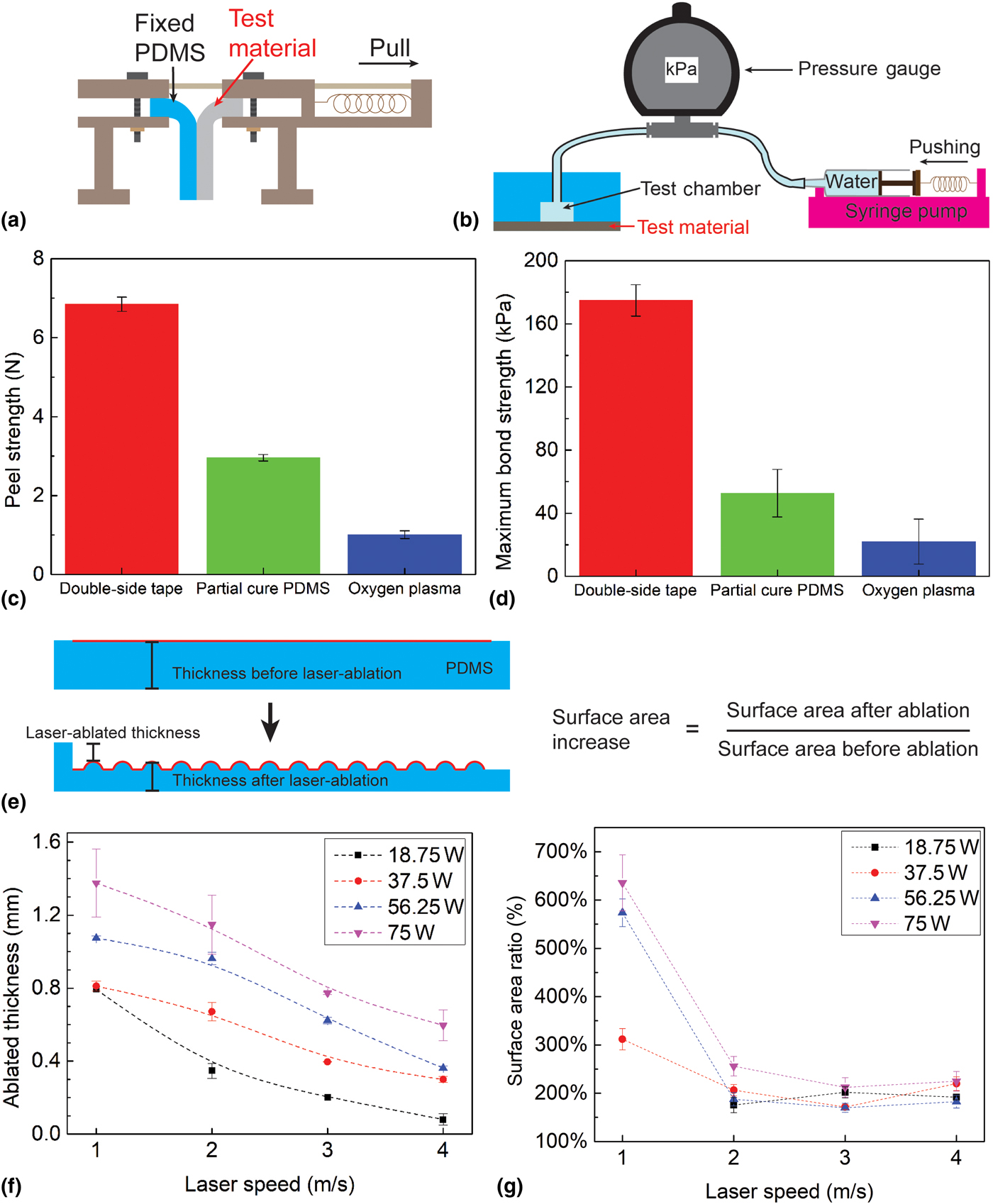
Figure 3. (a) and (c) Peel strength characterization among three bonding methods; (b) and (d) bond strength characterization among three bonding methods; (e) the conceptual illustration of laser-machined PDMS, including the definition of ablated/etched thickness and surface area ratio; and (f–g) the experiment results show both the ablated thickness and surface area ratio as a function of laser fabrication parameters (power and speed). Each data point represents the average of three trials.
The laser ablation characterization of PDMS was conducted by using a 10.6 µm wavelength CO2 laser system (PLS6MW, Universal Laser Systems, Inc.) to ablate a layer of 2 mm-thick PDMS. Ablation was conducted using various combinations of power (18.75–75 W) and scanning speed (1–4 m/s). The thickness of the ablated PDMS was defined as the sum of the amplitude of laser engineered roughness and the thickness of undamaged remaining PDMS; the surface area ratio was defined as the ratio of the surface area after and before the laser machining [red lines in Fig. 3(e)].
The oxygen permeability of the laser-ablated PDMS membrane was characterized by bonding the membrane to a PDMS chamber which was subsequently pressurized using oxygen gas [Fig. 4(a)]. The chamber was created by connecting a PDMS hollow cylinder (70 mm-thick, 80 mm outer diameter, 10 mm inner diameter) in series to a pressure gage and an oxygen pump. The test membrane (2.2 mm-thick, 80 mm diameter, with the central 10 mm diameter laser-ablated area) was then bonded to the cylinder. The laser-ablated area was <5.7% thinner than the rest of the chamber walls, thus ensuring the pressurized oxygen was released primarily through the ablated region. Finally, the pressure in the chamber was measured at various gas flow rates and was used to calculate the oxygen permeability of the laser-ablated regions. The experiments were conducted in triplicates using four different membrane thicknesses (0.3, 0.5, 0.9, and 2.1 mm), each with and without laser processing.
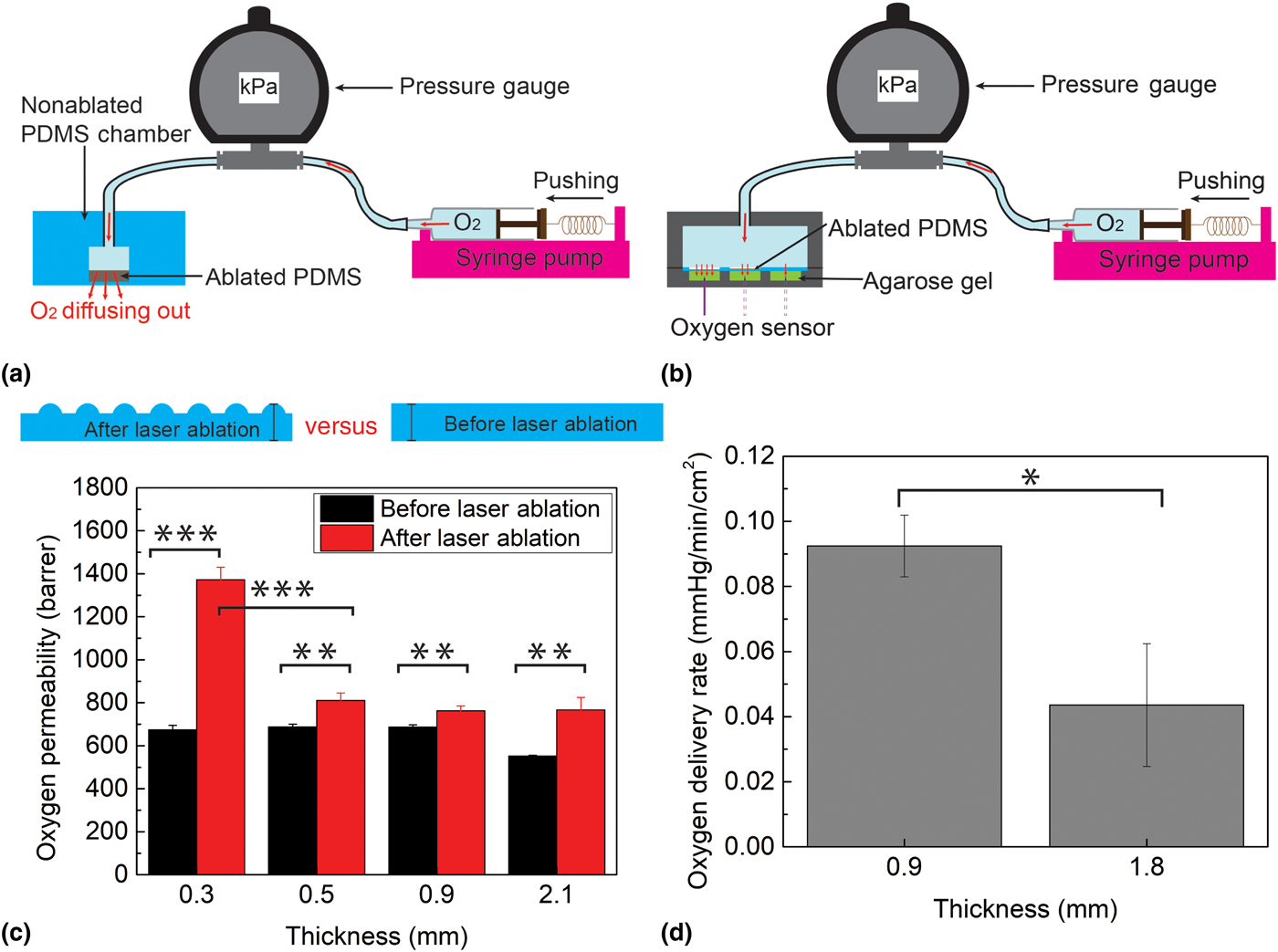
Figure 4. (a) Oxygen permeability experiment setup; (b) oxygen delivery experiment setup; (c) oxygen permeability comparison of laser-ablated PDMS and non-ablated PDMS at four different thicknesses, 0.3, 0.5, 0.9, and 2.1 mm; and (d) oxygen delivery capability comparison of laser-ablated PDMS at two thicknesses, 0.9 and 1.8 mm. Each data point represents the average of three trials. One asterisk (*) indicates P value smaller than 0.05 (P < 0.05); two asterisks (**) indicate P value smaller than 0.01 (P < 0.01); and three asterisks (***) indicate P value smaller than 0.001 (P < 0.001).
The oxygen delivery capability of the insole was investigated to determine the burst oxygen delivery rate during pressure ramp-up (representing transient pressure increase when the patient walks) as well as the long-time oxygen delivery rate at a small constant pressure (representing the patient sitting). A 2 mm-thick PDMS was laser-patterned to 0.9 and 1.8 mm-thicknesses (both on a 12 × 8 mm2 area) and sandwiched between two 3D-printed components. The top component served as an oxygen source container, and the bottom one consisted of three 12 × 8 × 9 mm3 cells, each containing a 0.35% agarose gel (mimicking three regions of the wound bed). A syringe pump was used to drive oxygen into the container at a controlled rate to create various pressures (150 and 2.28 kPa to represent walking and sitting, respectively) while simultaneously a fiber-optic oxygen measurement system (NeoFox, OceanOptics, Dunedin, FL) was used to measure the oxygen concentration in the agarose gel at a depth of 4 mm [Fig. 4(b)].
Results and discussion
A photograph of the fabricated insole (men size 7.5–8 shoe) with a selectively ablated pattern is shown in Figs. 2(d)–2(f), containing both the magnified top view and side views of the laser-engineered pattern. The photographed insole has an inner spatial volume of ~16.5 mL, and is thus able to store the same volume of oxygen under normal conditions (room temperature and atmosphere pressure); this capacity can be further upgraded by using a pressurized oxygen source. In addition, this entire fabrication process is scalable and can be adapted to commercial layer-by-layer equipment. The planar approach and specific materials used are essential for creating a design that is adaptable to various filling techniques, depending on industrial availability. For example, the insoles can be pre-filled during fabrication by evacuating the oxygen chamber with a hypodermic needle and subsequently filling them with pure oxygen gas, taking advantage of the self-sealing property of PDMS for small enough punctures.[Reference Keller, White and Sottos20] Alternatively, the insole can be loaded with un-reacted oxygenation precursors (e.g., hydrogen peroxide and a catalyst) that mix immediately prior to using it; for this, the insole can be modified to include microfluidic conduits for injecting the precursors and/or additional storage chambers that house the precursors until prior to usage. One such alternate is well-demonstrated by our group in terms of generating the oxygen at a laser-engineered position in a polymeric platform also targeting the wound healing application.[Reference Ochoa, Rahimi, Zhou, Jiang, Yoon, Oscai, Jain, Morken, Oliveira, Maddipatla, Narakathu, Campana, Zieger, Sood, Atashbar and Ziaie21]
The results of the peel strength tests are shown in Fig. 3(c), demonstrating the superior strength achieved using the adhesive transfer tape as compared with other methods. The tape method reaches an average maximum of 6.85 N peel strength, two times and six times larger than those of the partial cure PDMS and oxygen plasma techniques, respectively. Similarly, the results of the bond strength test show that the largest bond strength achievable using the adhesive transfer tape is 174.87 kPa, much larger than 52.67 or 22 kPa achievable when using the other two bonding techniques [Fig. 3(d)]. The mechanical strength results are particularly important since the bond must be able to withstand typical foot pressures (in the range of 120–200 kPa, corresponding to a range of patient's weight from 53 to 81 kg)[Reference Shu, Hua, Wang, Qiao Li, Feng and Tao22]; using the adhesive tape results in a sufficiently strong bond to meet this requirement. In addition, adhesive transfer tape also reduces the required bonding/annealing time. The insole is robust enough to be worn for at least one day (based on it showing no signs of integrity failure after being worn for 5160 steps over 8 h).
The results of the PDMS laser ablations are plotted in Figs. 3(f) and 3(g), showing a strong dependence of both the ablated thickness and surface area ratio on the laser power and processing speed. These relationships can be understood in terms of the energy imparted on the PDMS by the laser. With high power or low scanning speeds (slow movement of the laser head), the system imparts more energy (and hence more photo-thermal effect[Reference Klank, Kutter and Geschke18]) compared with using low power and high speeds. In response to the processing speed, the ablated thickness shows a linear relationship of 0.27 ± 0.04 mm/(m/s) (R 2 > 0.9), whereas the surface area ratio shows an exponential decrease. By selecting appropriate combinations of these parameters, it is possible to precisely tune the oxygen permeation rate to customize the patch for people in a broad range of weights and activity levels.
Figure 4(c) shows the results of the oxygen permeability experiments. Since the exact rate of release of oxygen depends on multiple factors (e.g., patient weight, foot area, foot deformities, and the presence of neuropathy[Reference Han and Ceilley3, Reference Howard, Asmis, Evans and Mustoe4]), in this proof-of-principle work we decided to characterize the aggregate effect of these parameters by considering the pressure exerted on the insole. For non-ablated PDMS, the data show an oxygen permeability of 552 barrer for 2.1 mm-thick PDMS and 680 barrer for thicknesses 0.9 mm or lower; these results agree with the published reports of PDMS oxygen permeability[Reference Merkel, Bondar, Nagai, Freeman and Pinnau23–Reference Lamberti, Marasso and Cocuzza25] and thus validate this experimental setup. The results demonstrate that the laser-ablation process increases the oxygen permeability for each original thickness (0.3, 0.5, 0.9, and 2.1 mm) by a statistically significant amount (P = 0.00002, 0.00235, 0.00285, and 0.00312). In particular, ablation causes a permeability increase of ~140 barrer (20.6% increase) when the thickness is above 0.5 mm, but as much as 1372 barrer (100% increase) when the thickness is 0.3 mm. This exponential increase in permeability with decreased thickness mimics the surface-to-volume trend observed in Fig. 3(g), suggesting that the permeability increase is primarily due to the effects of surface area. Whereas in the thicker membranes, oxygen transport kinetics are dominated by the thickness of the membrane (low surface-to-volume ratio), for the thinner sample, the surface area effects dominate. The results from these experiments demonstrate the effectiveness of laser-ablated PDMS for significantly increasing oxygen permeability, highlighting the potential for optimizing and tuning oxygen flux by engineering the surface/volume ratio of the membrane.
The burst oxygen delivery rate of the insole during a pressure transition (from 0 to 150 kPa) within first few minutes was measured as 1.8 ± 0.51 mmHg/min/cm2 using the 0.9 mm-thick laser-machined PDMS. This rate is comparable (slightly higher) with values in published reports of TO therapy for 30 min in a 2 × 2 cm2 window.[Reference Fries, Wallace, Roy, Kuppusamy, Bergdall, Gordillo, Melvin and Sen26, Reference Roe, Gibbins and Ladizinsky27] To measure the long-term oxygen delivery rate through a continuous oxygen diffusion into the gel, it is important to verify the delivery rate of oxygen when the insole is under low pressures (e.g., when the person is sitting or merely resting the foot, rather than standing/walking). To simulate this condition, oxygen was permeated through an ablated membrane under a constant pressure of 2.28 kPa. This pressure is 5% of the typical pressure exerted on the foot when a person is sitting (~47 kPa)[Reference Cheung and Zhang28]; therefore, oxygen delivery at this pressure serves as a lower bound for what a person would expect. The data show that with this setup, oxygen is delivered to the agarose gel at a rate of 0.092 mmHg/min/cm2 using 0.9 mm-thick PDMS, and at 0.044 mmHg/min/cm2 using 1.8 mm-thick PDMS (P = 0.014) [Fig. 4(d)]. The rate of 0.092 mmHg/min/cm2 is relevant, since at this rate, a 2 × 2 cm2 wound could be oxygenated to an efficacious concentration of 50 mmHg within a reasonable time period of 150 min.[Reference Hess, Howard and Attinger29] Thus, patients wearing the insoles can reliably oxygenate a foot ulcer by either standing, walking, or simply applying minimal pressure on their foot.
Overall, the insole described in this work has potential to provide oxygen to wounds with a clinically relevant rate, similar to that of commercial TO delivery systems. For instance, if we consider an insole with oxygen capacity of 16.5 mL, an ablated region of 0.3 mm thickness (providing oxygen permeability of 1372 barrer), a foot pressure of 150 kPa, and a typical wound area of 4 cm2, then the insole is expected to deliver oxygen at a flow rate of 7.44 mL/h, which is comparable to those (3–12 mL/h) of the commercial CDO commercial devices. Moreover, this oxygen delivery capability of the insole can be autonomously tuned by different applied pressures or wound sizes; for example, in order to supplement a wound bed of 1.6 cm2, the insole would maintain the delivery rate of 3 mL/h for 5.5 h. These values highlight the practicality of such an insole for foot ulcer treatment.
Conclusions
In this paper, we demonstrated a low-cost PDMS insole that can selectively deliver oxygen to diabetic ulcers on the sole of the foot. The top layer of the insole is ablated with a CO2 laser to create thinner regions with increased permeability to match the geometry of the wound. The insole shows good mechanical strength, allowing it to bear pressures close to 200 kPa (the mean foot pressure of an average person standing) and can withstand at least one day of wear (including 8 h of walking time with 5160 steps) without any structural failure. For typical standing/walking pressures, the insole can change the oxygen concentration in agarose gel (mimicking a wound) at the rate of 1.8 mmHg/min/cm2. At lower pressures (comparable with resting the foot while sitting), the oxygen concentration increases at 0.092 mmHg/min/cm2; this lower bound for the oxygenation rate would allow a wound to reach a therapeutic level of oxygen (50 mmHg) within 150 min. The insole can be customized to match each patient's wound location and geometry in order to promote optimal oxygen-supplemented wound healing.
Acknowledgments
The authors would like to thank the staff at Purdue University Birck Nanotechnology Center and the Ziaie Biomedical Microdevices Laboratory members for their assistance in fabrication and experiments. Funding for this project was provided by NextFlex PC 1.0 Project.