Article contents
Chemical and electronic structure analysis of a SrTiO3 (001)/p-Ge (001) hydrogen evolution photocathode
Published online by Cambridge University Press: 19 March 2018
Abstract
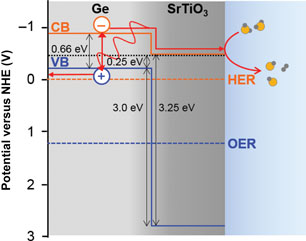
Germanium is a small-gap semiconductor that efficiently absorbs visible light, resulting in photoexcited electrons predicted to be sufficiently energetic to reduce H2O for H2 gas evolution. In order to protect the surface from corrosion and prevent surface charge recombination in contact with aqueous pH 7 electrolyte, we grew epitaxial SrTiO3 layers of different thicknesses on p-Ge (001) surfaces. Four-nanometer SrTiO3 allows photogenerated electrons to reach the surface and evolve H2 gas, while 13 nm SrTiO3 blocks these electrons. Ambient pressure x-ray photoelectron spectroscopy indicates that the surface readily dissociates H2O to form OH species, which may impact surface band bending.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
- 8
- Cited by



