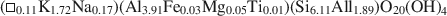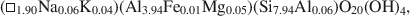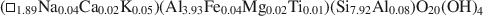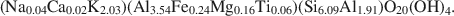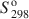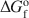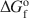Transmission electron microscopy has been used to characterize coexisting pyrophyllite and muscovite in low-grade metamorphosed pelites from Witwatersrand and northeastern Pennsylvania. The Witwatersrand sample consisted largely of porphyroblasts of interlayered muscovite and pyrophyllite in a fine-grained matrix of the same phases. In both textures, muscovite and pyrophyllite occurred as interlayered packets (with apparently coherent interfaces) from about 300 Å to a few micrometers in thickness, with no mixed layering. Their compositions were determined with a scanning transmission electron microscope to be$$(\Box{_{{\rm{0}}{\rm{.11}}}}{\rm{ }}{{\rm{K}}_{{\rm{1}}{\rm{.72}}}}{\rm{Na}_{{\rm{0}}{\rm{.17}}})(A}{{\rm{l}}_{{\rm{3}}{\rm{.91}}}}{\rm{ F}}{{\rm{e}}_{{\rm{0}}{\rm{.03}}}}{\rm{M}}{{\rm{g}}_{{\rm{0}}{\rm{.05}}}}{\rm{T}}{{\rm{i}}_{{\rm{0}}{\rm{.01}}}}{\rm{)(S}}{{\rm{i}}_{{\rm{6}}{\rm{.11}}}}{\rm{ Al}}{{\rm{l}}_{{\rm{1}}{\rm{.89}}}}{\rm{)}}{{\rm{O}}_{{\rm{20}}}}{{\rm{(OH)}}_{\rm{4}}}$$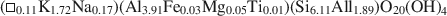
and$$(\Box_{{\rm{1}}{\rm{.90}}}{\rm{N}}{{\rm{a}}_{{\rm{0.06}}}}{{\rm{K}}_{{\rm{0}}{\rm{.04}}}}{\rm{)(A}}{{\rm{l}}_{{\rm{3}}{\rm{.94}}}}{\rm{F}}{{\rm{e}}_{{\rm{0}}{\rm{.01}}}}{\rm{M}}{{\rm{g}}_{{\rm{0}}{\rm{.05}}}}{\rm{)(S}}{{\rm{i}}_{{\rm{7}}{\rm{.94}}}}{\rm{A}}{{\rm{l}}_{{\rm{0}}{\rm{.06}}}}{\rm{)}}{{\rm{O}}_{{\rm{20}}}}{{\rm{(OH)}}_{\rm{4}}},$$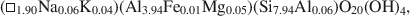
respectively.
The pyrophyllite and muscovite in the Pennsylvania shale likewise occurred only as coexisting coherent to sub-parallel packets as thin as 200 Å, with compositions of$$(\Box_{{\rm{1}}{\rm{.89}}}{\rm{N}}{{\rm{a}}_{{\rm{0.04}}}}{\rm{C}}{{\rm{a}}_{{\rm{0}}{\rm{.02}}}}{{\rm{K}}_{{\rm{0}}{\rm{.05}}}}{\rm{)(A}}{{\rm{l}}_{{\rm{3}}{\rm{.93}}}}{\rm{F}}{{\rm{e}}_{{\rm{0}}{\rm{.04}}}}{\rm{M}}{{\rm{g}}_{{\rm{0}}{\rm{.02}}}}{\rm{T}}{{\rm{i}}_{{\rm{0}}{\rm{.01}}}}{\rm{)(S}}{{\rm{i}}_{{\rm{7}}{\rm{.92}}}}{\rm{A}}{{\rm{l}}_{{\rm{0}}{\rm{.08}}}}{\rm{)}}{{\rm{O}}_{{\rm{20}}}}{{\rm{(OH)}}_{\rm{4}}}$$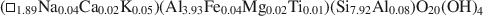
and$$({\rm{N}}{{\rm{a}}_{{\rm{0}}{\rm{.04}}}}{\rm{C}}{{\rm{a}}_{{\rm{0}}{\rm{.02}}}}{{\rm{K}}_{{\rm{2}}{\rm{.03}}}}{\rm{)(A}}{{\rm{l}}_{{\rm{3}}{\rm{.54}}}}{\rm{F}}{{\rm{e}}_{{\rm{0}}{\rm{.24}}}}{\rm{M}}{{\rm{g}}_{{\rm{0}}{\rm{.16}}}}{\rm{T}}{{\rm{i}}_{{\rm{0}}{\rm{.06}}}}{\rm{)(S}}{{\rm{i}}_{{\rm{6}}{\rm{.09}}}}{\rm{A}}{{\rm{l}}_{{\rm{1}}{\rm{.91}}}}{\rm{)}}{{\rm{O}}_{{\rm{20}}}}{{\rm{(OH)}}_{\rm{4}}}.$$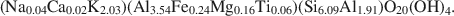
The textures of both samples were consistent with an equilibrium relationship between pyrophyllite and muscovite. The Pennsylvania sample also contained NH4-rich illite, kaolinite, and an illite-like phase having intermediate Na/K, which collectively imply non-equilibrated low-grade conditions.
The compositions of these coexisting pyrophyllite and muscovite define a solvus with steep limbs and extremely limited solid solution. Illite is a white mica, intermediate in composition between pyrophyllite and muscovite, formed at much lower temperatures than muscovite. These relations show that illite is metastable relative to pyrophyllite + muscovite in all of its diagenetic and low-grade metamorphic occurrences. This further implies that illite precursor phases, such as smectite, are also metastable. The prograde reactions involving smectite, illite, and muscovite are therefore inferred to represent Ostwald-step-rule-like advances through a series of metastable phases toward the equilibrium states attained in the greenschist facies. “Illite crystallinity” can therefore be a measure of reaction progress, for which temperature is only one of several determining factors.