Natural and synthetic micas have been used widely as substrates to study biological systems; but, as in the case of negatively charged DNA, anionic charge repulsion may render micas a less than ideal templating surface for many biological systems. The purpose of this study was to investigate the potential for the chlorite clinoclore, which contains a positively charged interlayer octahedral sheet, to serve as a substrate for DNA adsorption. The relationships between clinochlore cleavage characteristics, in terms of nano-morphology, and surface potential are investigated, as are its average crystal chemistry and topology. That the structural features of clinochlore can be used successfully to condense, order, and self assemble complex biomolecules, such as DNA is also proven.
A natural IIb-4 clinochlore [\$\end{document}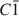 symmetry, unit-cell parameters a = 0.53301(4); b = 0.92511(6); c = 1.4348(1) (nm); α = 90.420(3); β = 97.509(3); γ = 89.996(4) (°)] with chemical composition \$\end{document}
symmetry, unit-cell parameters a = 0.53301(4); b = 0.92511(6); c = 1.4348(1) (nm); α = 90.420(3); β = 97.509(3); γ = 89.996(4) (°)] with chemical composition \$\end{document}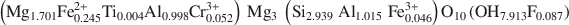 was selected. The octahedral sites of the silicate layer (<M(1)−O> = 0.2080 nm and <M(2)−O> = 0.2081 nm) are equal and occupied by Mg, whereas the octahedral sites in the interlayer M(3) and M(4) (<M(3)−O> = 0.2088 nm and <M(4) − O> = 0.1939 nm) show different sizes and are mostly completely occupied by divalent (Mg2+ and Fe2+) and trivalent (Al3+) cations, respectively.
was selected. The octahedral sites of the silicate layer (<M(1)−O> = 0.2080 nm and <M(2)−O> = 0.2081 nm) are equal and occupied by Mg, whereas the octahedral sites in the interlayer M(3) and M(4) (<M(3)−O> = 0.2088 nm and <M(4) − O> = 0.1939 nm) show different sizes and are mostly completely occupied by divalent (Mg2+ and Fe2+) and trivalent (Al3+) cations, respectively.
The clinochlore cleaved surface is present in two forms: (1) the stripe type (0.40 nm in height, up to several micrometers long and ranging from some nanometers to a few microns in lateral size); and (2) the triangular type (0.40 nm in height). Both features may result either from interlayer sheets whose cleavage weak directions are related to the different M(3) and M(4) site occupancy, or from weak interlayer bonding along specific directions to the 2:1 layer underneath. The cleaved surface, particularly at the cleaved edges, presents high DNA affinity, which is directly related to an average positive surface and ledge potential.