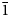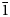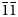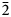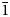Dongchuanite, ideally Pb4VIZnIVZn2(PO4)2(PO4)2(OH)2, is a new phosphate mineral with a new type of structure. It was found at the Dongchuan copper mine, Yunnan Province, People's Republic of China. Dongchuanite generally occurs as spherical aggregates with microscopic lamellar crystals, characterised by a turquoise–greenish blue colour. It is transparent, with a colourless streak and has a vitreous lustre without fluorescence. It is brittle with a Mohs hardness of 2–2½, and has good parallel cleavage to {011}, with insignificant parting and even fracture. According to the empirical formula and cell volume, it has a calculated density of 6.06 g/cm3. It easily dissolves in acid without gas being emitted. The mineral is biaxial (–), calculated n = 1.90 and maximum birefringence: δ = 0.010 and 2V=70°. Dispersion of the optical axes r < v is very weak. The mineral is pale blue to light blue and very weakly pleochroic in transmitted light. Dongchuanite crystallises in the triclinic space group P$\bar{1}$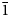 , with unit-cell parameters a = 4.7620(10) Å, b = 8.5070(20) Å, c = 10.3641(19) Å, α = 97.110(17)°, β = 101.465(17)°, γ = 92.273(18)°, V = 407.44(15) Å3 and Z = 1. The eight strongest reflections in the powder X-ray diffraction pattern [dobs, Å (I/I0) (hkl)] are: 3.442 (100) ($\bar{1}$
, with unit-cell parameters a = 4.7620(10) Å, b = 8.5070(20) Å, c = 10.3641(19) Å, α = 97.110(17)°, β = 101.465(17)°, γ = 92.273(18)°, V = 407.44(15) Å3 and Z = 1. The eight strongest reflections in the powder X-ray diffraction pattern [dobs, Å (I/I0) (hkl)] are: 3.442 (100) ($\bar{1}$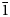 12), 3.035 (50) (120), 4.652 (45) (100), 2.923 (40) ($\bar{1}\bar{1}$
12), 3.035 (50) (120), 4.652 (45) (100), 2.923 (40) ($\bar{1}\bar{1}$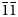 3), 2.384 (35) ($\bar{2}$
3), 2.384 (35) ($\bar{2}$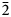 01), 3.130 (30) ($\bar{1}$
01), 3.130 (30) ($\bar{1}$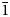 21), 2.811 (30) (030) and 2.316 (18) (032). The crystal structure (solved and refined from single-crystal X-ray diffraction data, R1 = 0.07) is a new layered structure consisting of corner-sharing tetrahedrons and octahedrons, where [PO4] tetrahedra and [ZnO4] tetrahedra share corners to form a double chain, and the another [PO4] tetrahedra is connected by corner-sharing with a [ZnO4(OH)2] octahedra to form a tetrahedral–octahedral chain, extending along the a-axis direction. The two types of chains are connected by corner-sharing between [ZnO4] and [PO4] tetrahedra forming a wrinkled layer parallel to (011). The Pb atoms occupy two independent sites between the wrinkled layers, both of which have typical lopsided coordination of Pb2+ with stereoactive 6s2 lone-pair electrons.
21), 2.811 (30) (030) and 2.316 (18) (032). The crystal structure (solved and refined from single-crystal X-ray diffraction data, R1 = 0.07) is a new layered structure consisting of corner-sharing tetrahedrons and octahedrons, where [PO4] tetrahedra and [ZnO4] tetrahedra share corners to form a double chain, and the another [PO4] tetrahedra is connected by corner-sharing with a [ZnO4(OH)2] octahedra to form a tetrahedral–octahedral chain, extending along the a-axis direction. The two types of chains are connected by corner-sharing between [ZnO4] and [PO4] tetrahedra forming a wrinkled layer parallel to (011). The Pb atoms occupy two independent sites between the wrinkled layers, both of which have typical lopsided coordination of Pb2+ with stereoactive 6s2 lone-pair electrons.