Compreignacite, a naturally occurring potassium uranyl oxide hydrate, is an expected alteration product of spent nuclear fuel subjected to oxidative corrosion in the presence of water. Ion-exchange experiments were performed using natural crystals of compreignacite in a 2 M CsCl solution at 180°C for 24 h, and in a 100 ppm CsCl solution at 90°C for 14 days. Exchange of Cs into crystals of compreignacite was demonstrated by crystal-structure analysis for a crystal from 2 M solution and chemical analysis for crystals from both exchange experiments. The structure of Cs-exchanged compreignacite is hexagonal, space group P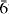 2m, a= 14.1014 (18) Å, c = 15.127(3) Å, V = 2605.1(7) Å2. It was solved by direct methods and refined on the basis of F2 for all unique reflections to a final R1 = 6.37%. The structure determination demonstrated almost complete exchange of Cs for K in the interlayer of the structure, as confirmed by compositional analysis of the crystals, and provided the formula Cs6[(UO2)12(OH)10O10](H2O)3.5, Z= 2. The structure of Cs-exchanged compreignacite contains α-U3O8-type sheets of uranyl pentagonal bipyramids that are topologically identical, although compositionally distinct, to those in compreignacite. Compreignacite crystals placed in 100 ppm CsCl solution also incorporated substantial Cs by ion exchange. Ion exchange experiments, using Cs-exchanged compreignacite from earlier experiments, in 2 M KCl solution at 90°C for 14 days showed that compreignacite will retain significant Cs in the presence of a solution rich in K. These experiments indicate that formation of compreignacite structure-type phases in a geological repository due to alteration of nuclear waste may significantly impact upon the mobility of Cs, either by direct incorporation of Cs into growing crystals, or by exchange of Cs into earlier-formed crystals of compreignacite when they contact Cs-bearing solutions.
2m, a= 14.1014 (18) Å, c = 15.127(3) Å, V = 2605.1(7) Å2. It was solved by direct methods and refined on the basis of F2 for all unique reflections to a final R1 = 6.37%. The structure determination demonstrated almost complete exchange of Cs for K in the interlayer of the structure, as confirmed by compositional analysis of the crystals, and provided the formula Cs6[(UO2)12(OH)10O10](H2O)3.5, Z= 2. The structure of Cs-exchanged compreignacite contains α-U3O8-type sheets of uranyl pentagonal bipyramids that are topologically identical, although compositionally distinct, to those in compreignacite. Compreignacite crystals placed in 100 ppm CsCl solution also incorporated substantial Cs by ion exchange. Ion exchange experiments, using Cs-exchanged compreignacite from earlier experiments, in 2 M KCl solution at 90°C for 14 days showed that compreignacite will retain significant Cs in the presence of a solution rich in K. These experiments indicate that formation of compreignacite structure-type phases in a geological repository due to alteration of nuclear waste may significantly impact upon the mobility of Cs, either by direct incorporation of Cs into growing crystals, or by exchange of Cs into earlier-formed crystals of compreignacite when they contact Cs-bearing solutions.

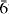 2m,
2m, 