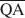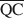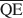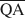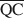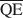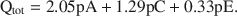The formation of an interstratified structure in dioctahedral smectite was assumed to be influenced by (1) the overall layer charge density and its distribution in the structure, (2) the solvation energy of the cation, and (3) the nature of the solvation agent. By holding factors (2) and (3) constant it was possible to calculate the average local charge densities $\overline {{\rm{QA}}}$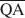 , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $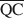 , and $\overline {{\rm{QE}}} $
, and $\overline {{\rm{QE}}} $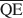 which are necessary for formation of 10-, 14-, and 16.8-Å mixed-layer phases in potassium-treated and ethylene glycol (EG) saturated smectites. The values of $\overline {{\rm{QA}}} $
which are necessary for formation of 10-, 14-, and 16.8-Å mixed-layer phases in potassium-treated and ethylene glycol (EG) saturated smectites. The values of $\overline {{\rm{QA}}} $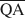 , $\overline {{\rm{QC}}} $
, $\overline {{\rm{QC}}} $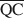 , and $\overline {{\rm{QE}}} $
, and $\overline {{\rm{QE}}} $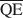 were 1.99, 1.2, and 0.56 esu/unit cell, respectively. Ammonium-treated smectites saturated with EG gave corresponding mean local charge densities of 2.7, 1.6, and 0.72 esu/unit cell. Calculations were made under the limiting condition QA > QC > QE > 0.
were 1.99, 1.2, and 0.56 esu/unit cell, respectively. Ammonium-treated smectites saturated with EG gave corresponding mean local charge densities of 2.7, 1.6, and 0.72 esu/unit cell. Calculations were made under the limiting condition QA > QC > QE > 0.
For K-smectites saturated with EG, Qtot = 1.99pA + 1.2pC + 0.56pE, where Qtot is the total charge (esu/unit cell), and pA, pC, and pE are probability coefficients for 10-, 14-, and 16.8-Å phases in the interstratified structure. The above equation calculated with the aid of least squares and without the limiting condition yields$${{\rm{Q}}_{{\rm{tot}}}} = {\rm{2}}{\rm{.05pA + 1}}{\rm{.29pC + 0}}{\rm{.33pE}}{\rm{.}}$$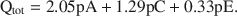
There is a good agreement between values obtained for K-smectites and those for mica, vermiculite, and montmorillonite layer charges for which the above unit-structure distances are typical.