INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) causes an estimated 840 million gastrointestinal infections [Reference Wenneras and Erling1], and about 380 000 deaths worldwide each year [2]. Infection with ETEC leads to profuse watery diarrhoea, and can be confused with cholera. ETEC is primarily spread through food or water contaminated with human waste, and is a frequent cause of outbreaks of diarrhoea. Prevention and control of ETEC infections is through the provision of safe food, water and sanitation, which has proved difficult to achieve on a large scale in the developing world. Currently no effective vaccine exists.
To identify contemporary gaps in the understanding of the global epidemiology of ETEC infection, we conducted a review of the recent scientific literature. We summarize below recent available data on morbidity and mortality burden, the age, geographic and temporal distribution of ETEC, the isolation of ETEC in relation to other diarrhoeagenic pathogens, and the frequency of the different ETEC toxin types. To provide context to its global epidemiology, we also describe ETEC diagnostics and preventive measures. Data gaps and existing research needs are then discussed.
BACKGROUND
Bacteriology
As members of the family Enterobacteriaceae, Escherichia coli are mostly Gram-negative, non-spore-forming, motile, lactose-fermenting rods. Although most E. coli are non-pathogenic commensal organisms that colonize animal and human intestines, some highly adapted strains can cause human illness.
Enterotoxigenic E. coli (ETEC), a cause of watery diarrhoea, belong to a large number of serotypes (defined by the lipopolysaccharide O antigen, flagellar H antigen and capsular K antigen types). They are defined by the ability to produce the heat-labile toxin (LT), heat-stable toxin (ST), or both (LT/ST). More than 20 colonization factors (CFs), found in adhesive fimbriae or bacterial cell surfaces, have also been identified. Immunity is poorly understood, but antibodies to both serotype-specific antigens and LT are thought to confer protective immunity [Reference Boedeker3]. Although a single individual can suffer repeated ETEC infections, persons living in endemic areas and exposed to multiple strains often develop immunity to a broad range of ETEC strains.
The infectious dose of ETEC is high, and estimated to range from 106 to 1010 organisms [Reference Levine4], although vulnerable populations such as children and the elderly may be susceptible to infection at lower doses. In order to cause diarrhoea, small intestine colonization, enterotoxin elaboration and action on enterocytes must all occur. The CFs promote small intestine colonization. Secretory watery diarrhoea can be caused by either LT or ST, both of which stimulate chloride secretion and inhibit chloride absorption in small intestine epithelial cells. LT is structurally and functionally similar to cholera toxin; ST is a very small protein that does not appear to elicit an immune response. The role of different CFs in determining severity of illness is poorly understood [Reference Gothefors5, Reference Katouli6].
The effect of antimicrobial use on the course of illness caused by ETEC is poorly understood, as diagnosis of ETEC is expensive and not readily available to most clinicians. Antimicrobial resistance to drugs such as ampicillin and trimethoprim–sulfamethoxazole is common. If antimicrobial agents are used, fluoroquinolones such as ciprofloxacin or norfloxacin are typically recommended [Reference Heyman7, Reference Mattila8], although resistance to these antimicrobials has been documented as well [Reference Chakraborty9], and their use in children is controversial.
Clinical features
The incubation period of ETEC is typically between 10 h and 3 days. Illnesses typically last for 3–5 days; shorter or longer duration illness can also occur [Reference Dalton10]. ETEC infection is characterized by watery diarrhoea with either no fever or a low grade fever, but symptoms can also include abdominal cramping and vomiting. Severe, life-threatening dehydration can occur. Extraintestinal complications, such as bacteraemia, are not generally seen with ETEC infections.
Role of environmental and social factors
ETEC is found in both animal and human intestines. Although virulence factors, such as CFs and LT and ST subtypes, and infecting strains are generally species-specific [Reference Porat11, Reference Steinsland12], animal strains have been reported to cause illness in humans [Reference Nishikawa13]. Porcine ST toxin (STp) causes disease in humans, and diagnostic methods which differentiate STp from human ST toxin (STh) may help identify differences in the epidemiology of these two strains [Reference Steinsland14]. Unlike Shigellae or E. coli O157:H7, the high infectious dose of ETEC makes direct person-to-person spread rare. Instead, ETEC are primarily transmitted through contaminated food and water. In young infants who may become ill with a lower infectious dose than adults, the introduction of water or weaning foods has been shown to lead to ETEC infections as early as age 3 months [Reference Long15]. Poor drinking water quality, lack of a sewage system and feeding of supplementary foods were found to be risk factors for ETEC infection in a study of Ecuadorian infants [Reference Brussow, Rahim and Freire16]. In Bangladesh, ETEC has also been found to be a major cause of epidemic watery diarrhoea during floods [Reference Qadri17]. Countries with the highest incidence of ETEC infection tend to have rapid population growth, periurban areas with poor infrastructure combined with rapid urbanization, sewage-contaminated drinking water and poor sanitary facilities. The epidemiology of ETEC is therefore related to poor socioeconomic conditions.
METHODS
We systematically searched the English-language scientific literature published between 1984 and 2005, using the Medline database, restricting the search to low and medium development countries according to the Human Development Index, United Nations Development Programme (accessed at www.hdr.undp.org). A set of articles containing the key words epidemiol-, morbidity, mortality, disease outbreaks, incidence, prevalence, seasons, population surveillance, age distribution, and longitudinal survey was linked with a set of pathogen-specific terms (Table 1). The resulting cross-linked set was reviewed for publications that contained information addressing specific questions on morbidity, mortality, age distribution, geographic distribution, temporal distribution, pathogen-specific preventive measures, and diagnostics. Population-based studies with culture confirmation of cases were considered primary data sources. When these were lacking, hospital-based studies were considered. Publications were then evaluated for their contribution to an understanding of the global epidemiology of ETEC, and gaps in the data were identified.
Table 1. Terms used in literature search to identify gaps in data on enteric disease burden

Publications were sought with information on the incidence of the infection by region according to the 21 regions of the United Nations Department of Social and Economic Affairs, Population Division (http://esa.un.org/unpp/). We examined the correlation between the incidence of ETEC infection and the national per capita gross domestic product, adjusted for purchasing power parity in year 2000 dollars, at the time that the studies were conducted (http://unstats.unm.org/unsd/cdb/cdb_series_xrxx.asp?series_code=29922). We searched for publications with information on the age-specific incidence, morbidity, seasonality, mortality, preventive measures and diagnostics. We examined the frequency of ETEC relative to other diarrhoea-causing pathogens including Salmonella, Shigella, Vibrio cholerae O1, Campylobacter and Rotavirus. In studies in which stools of healthy controls were tested in addition to those with diarrhoea, we report frequency of ETEC isolation only among those with diarrhoea, but discuss the impact of asymptomatic infection on estimates of disease incidence separately.
RESULTS
Morbidity
Incidence
In a 2004 review, the annual number of ETEC cases in the developing world was estimated at 840 million episodes, with another 50 million asymptomatic carriers in children aged <5 years [Reference Wenneras and Erling1]. Incidence of symptomatic infections was estimated at 500 cases/1000 person-years for children aged <5 years, and 100 cases/1000 person-years for older persons. These figures were derived by estimating the total number of diarrhoeal cases and multiplying that by the proportion attributable to ETEC, as determined by the yield of ETEC in stool among persons with diarrhoea found in a variety of studies and age groups.
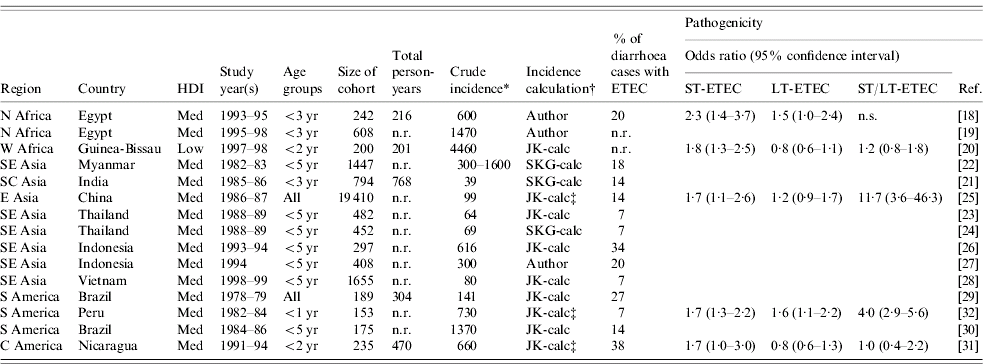
In this review of the published literature from 1984 to 2005, we found 15 population-based studies of ETEC incidence in low and medium human development index (HDI) countries (Table 2) [Reference Abu-Elyazeed18–Reference Black32]. Fourteen of these studies were conducted in ten medium-HDI countries, representing Asia (8), Africa (2), and Latin America and the Caribbean (4). The fifteenth study was conducted in a low-HDI country, Guinea–Bissau.
Table 2. Population-based studies of ETEC incidence published 1984–2005
ETEC, Enterotoxigenic E. coli; HDI, human development index; ST, heat-stable toxin; LT, heat-labile toxin; n.r., not reported; n.s., data not sufficient.
* Crude incidence reported as number of cases/1000 persons per year.
† Author: incidence reported by author of paper; JK-calc or SKG-calc: incidence calculated by the investigators.
‡ Odds ratios for toxin types calculated using Epi-Info v.3.3.2, based on numbers of ill/well individuals with positive/negative toxin tests.
The incidence data presented in Table 2 were reported by the authors of three studies and calculated by the investigators based on data provided for the other 12 studies. Crude incidence estimates are given, without correction for age categories, sensitivity of diagnostic methods for ETEC, asymptomatic or LT-ETEC infection, or for the proportion of diarrhoeal episodes for which stool specimens were not collected. The study methodologies varied widely and incidence results are not comparable. In these 15 studies, longitudinal surveillance visits were conducted daily (3), three times a week (2), twice a week (5), weekly (4) or monthly (1). Eleven different definitions of diarrhoea were used and seven different algorithms were used for identification of ST and LT enterotoxins.
Among the two studies reporting on surveillance among all age groups, from China and Brazil, ETEC incidence was similar, 99 and 141 cases/1000 person-years. The other 13 studies included different subsets of children aged <5 years, and showed a wide range of incidence (39–4460 cases/1000 person-years), with no clear pattern by region, HDI level or population setting.
We did not find any published population-based studies of ETEC incidence from any country in sub-Saharan Africa or West Asia.
Age distribution
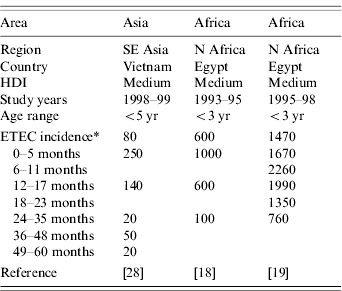
Neither of the two population-based studies of ETEC disease incidence which included all age groups reported age-specific incidence [Reference Chen25, Reference Giugliano29]. However, three population-based studies of ETEC disease incidence among children only reported age-specific incidence (Table 3). All three studies showed the same pattern of peak incidence in the first year of life, with subsequent declines in incidence (Fig.). Rao et al. stratified infants by age and showed that the peak incidence is after 6 months and may last until age 18 months [Reference Rao19]. Children aged >6 months may be at increased risk due to reduced levels of maternal antibodies and increased consumption of contaminated water or foods.
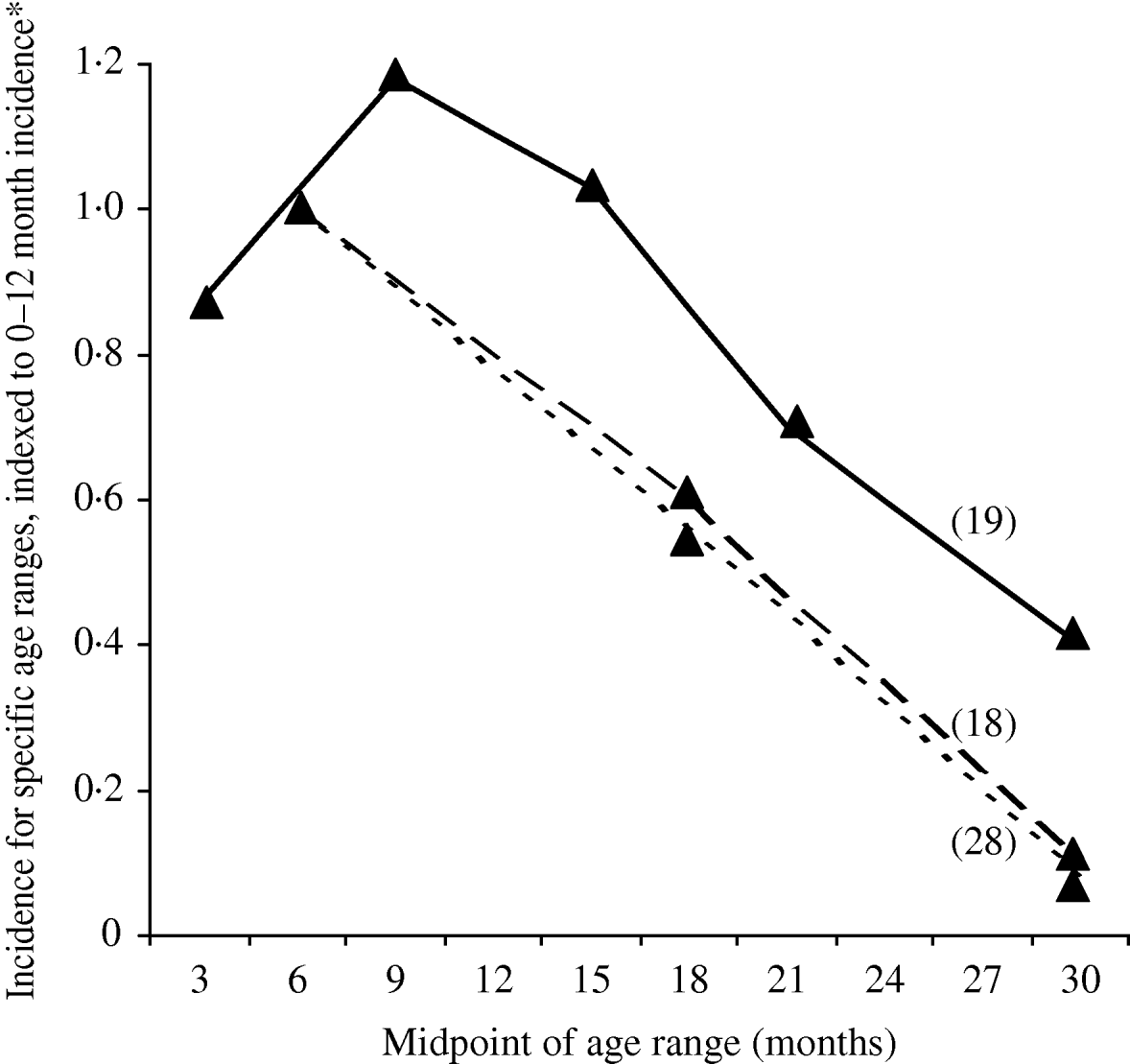
Fig. Age-specific incidence of ETEC infections in population-based studies, published 1984–2005. * In order to compare results in one figure, incidence is expressed as the incidence for the indicated age group, divided by the incidence for infants aged 0–12 months.
Table 3. Age-specific incidence of ETEC infections in population-based studies, published 1984–2005
ETEC, Enterotoxigenic E. coli; HDI, human development index.
* Incidence reported as number of episodes/1000 person-years.
Temporal distribution
Seventeen studies reported seasonality of ETEC infections. Of these, three reported no seasonal variation [Reference Black32–Reference Lindblom34]. Another ten reported a higher incidence in the hot season [Reference Steinsland12, Reference Steinsland22, Reference Steinsland35–Reference Khan42]. The other four studies reported that ST-ETEC had a higher incidence in the hot season, but LT-ETEC showed no seasonal variation [Reference Abu-Elyazeed18, Reference Rao19, Reference Yamashiro43, Reference Subekti44]. No consistent patterns were seen when comparing wet and dry seasons.
Geographic distribution
The geographic distribution of ETEC is illustrated based on data from population-based studies presented in Table 2. Incidence ranges from 39 to 4460 cases/1000 person-years. Results from these specific study sites cannot be generalized to an entire country or geographic region. These rates are considerably higher than most other bacterial enteric infection rates, such as Shigella [Reference Ram45] and Salmonella Typhi [Reference Crump46]. No data were available for Eastern, Middle and Southern Africa and Western Asia. The highest ETEC incidence was reported from North and West Africa.
Frequency of ETEC relative to other diarrhoeagenic pathogens
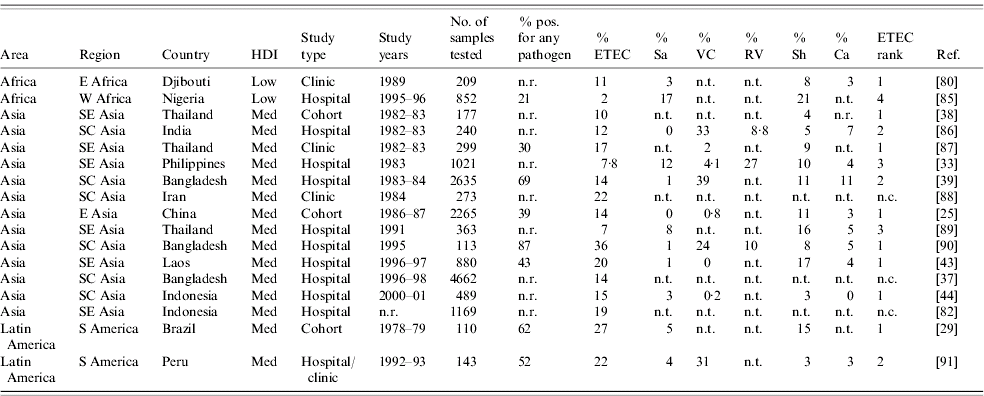
We found 70 publications that described the frequency of ETEC in diarrhoea stools or the relative frequency of ETEC compared to other enteric pathogens. Table 4 summarizes these publications and includes the age of the population surveyed, the total number of stool samples or rectal swabs obtained, and where reported, the total proportion of samples yielding at least one pathogen. Studies varied widely with respect to the overall spectrum of pathogens tested. When available, the proportion of isolates yielding Salmonella, Vibrio cholerae, ETEC, Campylobacter, Rotavirus, and Shigella are reported. ETEC is assigned a rank among the pathogens based on data presented in Table 4 as well as data regarding other pathogens, such as enteropathogenic E. coli, which may have been presented in the studies under consideration.
Table 4a. Relative frequency of endemic ETEC isolation, community- and facility-based studies conducted among all age groups, published 1984–2005
ETEC, Enterotoxigenic E. coli; HDI, human development index; Sa, Salmonella (includes typhoidal and non-typhoidal serotypes); VC, Vibrio cholerae; RV, Rotavirus numbers; Sh, Shigella; Ca, Campylobacter; n.r., not reported; n.t., not tested; n.c., not calculable.
Table 4b. Relative endemic ETEC isolation frequency, community- and facility-based studies conducted among restricted age groups, published 1984–2005
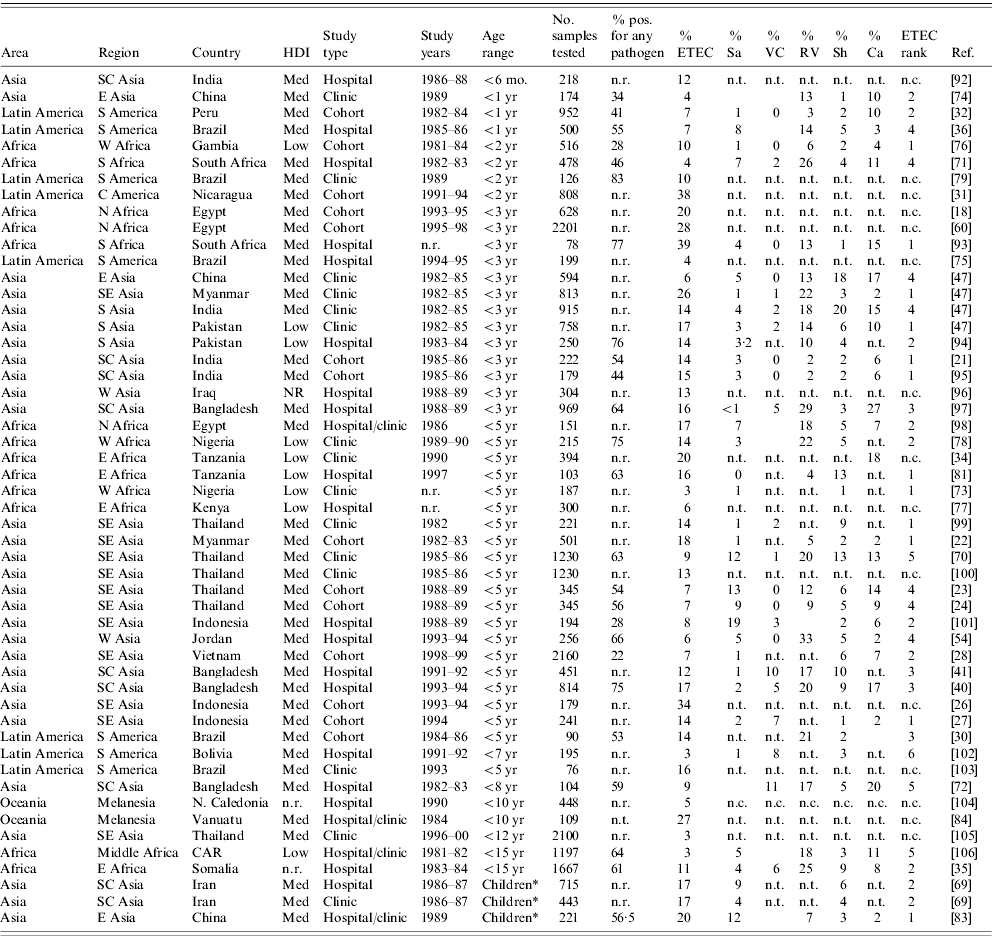
ETEC, Enterotoxigenic E. coli; HDI, human development index; Sa, Salmonella (includes typhoidal and non-typhoidal serotypes); VC, Vibrio cholerae; RV, Rotavirus; Sh, Shigella; Ca, Campylobacter; CAR, Central African Republic; n.r., not reported; n.t., not tested; n.c., not calculable.
* Children: the corresponding study did not describe the age range of children included in the study.
The 70 studies represent Asia (42), Africa (16), Latin America and the Caribbean (10) and Oceania (2). One study was conducted in four different low- or medium-HDI countries [Reference Huilan47] and is considered here as four distinct studies. Of 52 studies with relative frequency of pathogens other than ETEC, ETEC was the most frequently isolated pathogen in 21 (40%) studies and the second most frequently isolated in another 14 (27%) studies. ETEC was the first or second most frequently isolated pathogen in nine (75%) of the 12 studies conducted in Africa, 22 (65%) of 34 studies in Asia and three (50%) of six studies in Latin America and the Caribbean. Notably, only 37 (73%) of the 51 studies tested for and reported results of Campylobacter isolation, and only 32 (63%) tested for and reported results of Rotavirus infection. Most studies did not include identification of parasitic aetiologies of diarrhoea.
Among the 19 studies conducted with all age groups, a median of 14% of diarrhoeal specimens were positive for ETEC (range 2–36%). Among the 51 studies conducted among children only, a median of 13% of diarrhoeal episodes were positive for ETEC (interquartile range 3–39%).
Frequency of ETEC toxin phenotypic subgroups
Knowledge of the distribution of ETEC toxin phenotypic subgroups in a population may be useful to assess endemic disease incidence, as LT-ETEC is thought to be less likely to cause disease than ST-ETEC or LT/ST-ETEC [Reference Qadri48]. Pathogenicity of LT-ETEC may be in part determined by the presence of specific CFs. The literature review yielded a total of 50 studies assessing the relative frequency of the three ETEC enterotoxin subgroups (Table 5). ST-ETEC was the most commonly detected subgroup in 27 of these, LT-ETEC in another 19, and ST/LT-ETEC in the remaining four. There was wide variability in the frequency of toxin phenotypic subgroups among ETEC isolates. The range (median) of frequency was 1–98% (30%) for LT-ETEC, 0–92% (45%) for ST-ETEC, and 0–52% (14%) for LT/ST-ETEC. This wide variation was seen in Africa, Asia and Latin America. No relationship between relative frequency of toxin phenotypic subgroups and study age group, year of study or study setting was noted.
Table 5. Relative frequency of ETEC enterotoxin subgroups in endemic disease, published 1984–2005
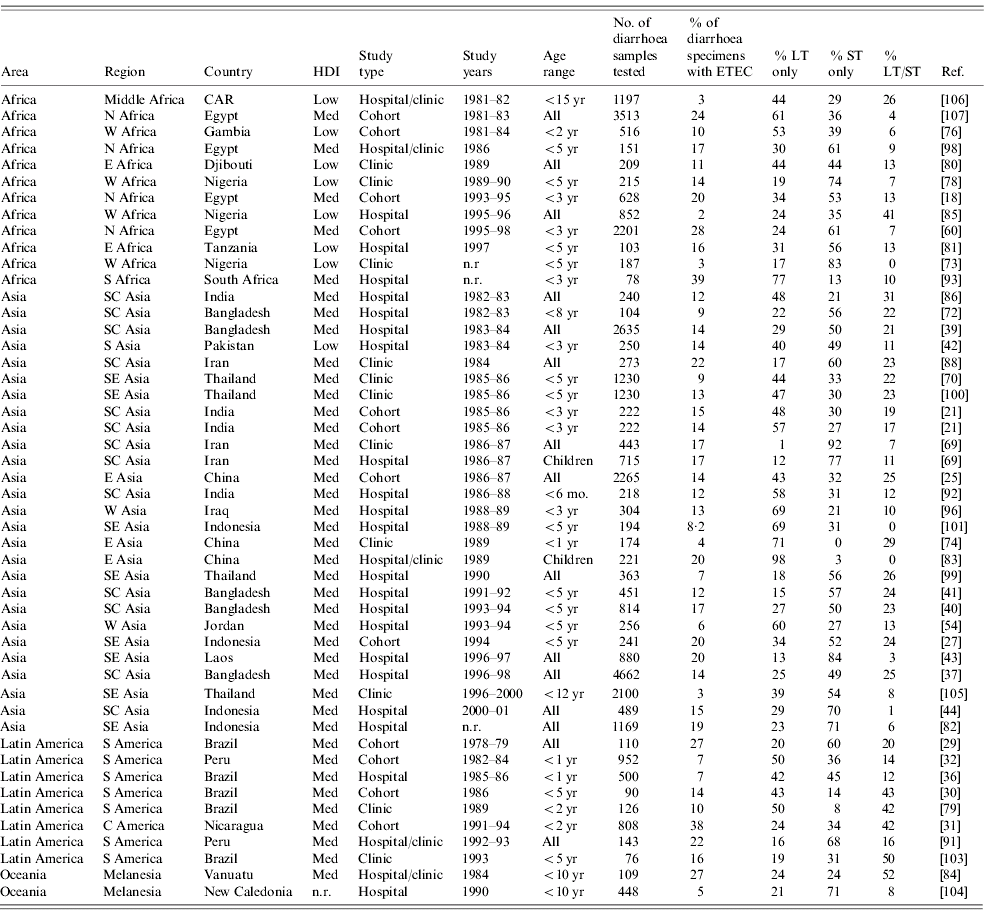
ETEC, Enterotoxigenic E. coli; HDI, human development index; LT, heat-labile toxin; ST, heat-stable toxin; CAR, Central African Republic; n.r., not reported.
Five of the 15 population-based studies of ETEC incidence obtained sufficient data to determine the pathogenicity of different toxin types (Table 2) [Reference Abu-Elyazeed18, Reference Valentiner-Branth20, Reference Chen25, Reference Paniagua31, Reference Black32]. In all five of these studies, ill patients were more likely to have ST-ETEC isolated from their stools than well patients. However, in only two of five studies LT-ETEC was more likely to be isolated from ill patients, and in two or four studies ST/LT-ETEC was more likely to be isolated from ill patients. These results are consistent with facility-based case-control studies which suggest that LT-ETEC, or certain strains of LT-ETEC may be infrequently pathogenic [Reference Qadri48].
Three studies described the relative frequency of toxin phenotypic subgroups during three different ETEC outbreaks in India [Reference Chakraborty9, Reference Kulshrestha49, Reference Taneja50]. All ETEC isolates from each outbreak were positive for LT and negative for ST, suggesting that LT-ETEC does cause clinical illness. Outbreaks of watery diarrhoea due to ETEC may often go unrecognized because of the unavailability of tests for LT and ST outside research settings.
Complications
Extraintestinal complications do not generally occur with ETEC infections. The most concerning acute complication is severe dehydration due to profuse watery diarrhoea and vomiting. In a hospital-based study in Bangladesh from 1996 to 2002, 36% of 478 adults and 3% of 1107 children admitted with ETEC infection had severe dehydration [Reference Qadri48]. This is consistent with reports that adults, at least in the Indian subcontinent, frequently present with a severe dehydrating cholera-like illness, although this also could be due to longer delays in reaching a treatment facility [Reference Qadri37]. In India, one outbreak of severe watery diarrhoea thought to be due to cholera was found to be due to ETEC [Reference Kulshrestha49] and another was found to be due to both cholera and ETEC [Reference Chakraborty9].
ETEC infections may also cause malnutrition and poor weight gain. In one study in rural Bangladesh, ETEC infections were shown to decrease 60-day weight gain in children [Reference Black, Brown and Becker51]. In Brazil, childhood diarrhoea was shown to be associated with long-term impaired physical fitness and cognitive function [Reference Guerrant52], although this has not been studied specifically with ETEC infections.
Mortality
Annual diarrhoeal deaths among children aged <5 years are estimated at 2·5 million based on studies conducted between 1992 and 2000 [Reference Kosek, Bern and Guerrant53]. If ETEC is responsible for 13% of these deaths, the median proportion of ETEC among diarrhoea cases in this review, 325 000 children (interquartile range 175 000–425 000) aged <5 years would be estimated to die each year from ETEC.
Of the 15 population-based studies of ETEC, only two discuss mortality. In Vietnam, 2160 cases of diarrhoea were identified over one year in 1655 children aged <5 years; of 25 ETEC cases, there were no deaths [Reference Isenbarger28]. In Nicaragua, 885 cases of diarrhoea were reported in the first two years of life among 235 children; of 310 ETEC cases detected among 808 specimens, there were no deaths [Reference Paniagua31]. Of the hospital-based studies of endemic disease referenced in this report, one discusses mortality; among 265 children admitted with acute diarrhoea, none died [Reference Youssef54]. In three epidemics attributed to ETEC in India [Reference Chakraborty9, Reference Kulshrestha49, Reference Taneja50], no patient deaths were reported. If patients with severe ETEC infections reach a treatment facility, the mortality rate should be extremely low. However, most infections may go untreated, and the case-fatality rate (CFR) for untreated infections is unknown.
Pathogen-specific preventive measures
Vaccine strategies
Efforts to develop an effective and useful ETEC vaccine are ongoing. Many different antigens have been considered in vaccine development including O antigens, H antigens, CFs and toxins. At least 78 O antigens and 34 H antigens have been identified in ETEC isolates, with no predominant serotype or group of serotypes [Reference Wolf55, Reference Peruski56]. O and H antigens are known to confer protective immunity [Reference Rivas57], and infection with multiple serotypes is believed to confer immunity to the predominant ETEC strains present in a community. However, because of the large number of O and H antigens, these have not been considered practical targets for vaccine development unless common epitopes can be identified [Reference Boedeker3, Reference Wolf55]. The ST toxin is not immunogenic because of its small size (only 19 amino acids), and efforts to design a ST-conjugate antigen that confers protective immunity against ST-ETEC have not yet been successful [Reference Peruski56].
A 1988 study showed 67% short-term, 3-month protection against illness from LT-ETEC by a B subunit whole-cell oral cholera vaccine [Reference Clemens58], providing hope for the development of an ETEC vaccine. Current experimental ETEC vaccines currently target the LT toxin as well as some of the CFs [Reference Boedeker3]. However, this strategy is limited because of the small proportion of ETEC illnesses attributable to LT-ETEC, the large number of CFs, the high proportion of ETEC isolates without identifiable CFs, the finding that CFs may not effectively confer protective immunity [Reference Steinsland59], and the lack of effectiveness of a candidate vaccine in a recent trial [Reference Clemens60]. The success of future vaccine development efforts will depend on the ability to find or design the best vaccine antigens for protective immunity, and to find effective methods of vaccine delivery for optimal antigen presentation [61].
Non-vaccine strategies
Both young age and pre-existing malnutrition have been found to be associated with more severe ETEC infections [Reference Black, Brown and Becker62, Reference Black63]. The effect of micronutrient deficiencies such as vitamin A and zinc on risk for severe ETEC infection is unknown. Exclusive breastfeeding of infants has been shown to protect against severe ETEC infections, but this effect was not seen with partial breastfeeding or with children aged >1 year [Reference Clemens64].
Non-vaccine measures for the prevention of ETEC include exclusive breastfeeding of infants [Reference Clemens64, Reference Long65], sanitary preparation of weaning foods for infants [Reference Long65], promotion of handwashing and hygiene, control of houseflies [Reference Khin New, Sebastian and Aye66], sanitary disposal of human faeces, provision of safe drinking water at the point of consumption through improved water supplies, household water disinfection and safe water storage [Reference Daniels67], sanitary food preparation and storage [Reference Dalton10], and the provision of safe drinking water during flood disasters [Reference Qadri68].
Diagnostics
Testing for both LT and ST in E. coli isolates is the established method for the identification of ETEC. Traditionally, enterotoxins have been detected with immunoassays or bioassays. Because of the large number of serotypes associated with ETEC, serotyping is not as useful for diagnosis. Because it is faster, detection of the LT or ST gene through the use of DNA probes or PCR amplification has become common. In incidence studies, the differences in sensitivity of different diagnostic methods may partially explain the wide variety in incidence estimates, relative frequency of ETEC in diarrhoeal stools and relative frequency of ETEC toxin types. However, as all ETEC diagnostic methods are widely unavailable in most clinical laboratories in both the developing and developed world, ETEC is likely under-recognized outside of research settings.
DATA GAPS AND DISCUSSION
This review describes the available data and highlights the major gaps in data (below) regarding the global epidemiology of ETEC infections. A successful research agenda based on these data gaps could be expected to help appropriately direct resources for prevention and control activities in order to more efficiently reduce the burden of illness due to ETEC. This research can be performed for multiple diarrhoeagenic pathogens simultaneously in order to both reduce costs and better understand the relative contribution of different pathogens to age-specific morbidity and mortality.
The major data needs for incidence, including age, temporal and geographic distribution are:
• population-based surveillance for ETEC incidence in low-HDI countries, especially in sub-Saharan Africa, including testing in both ill and well participants;
• population-based surveillance, with incidence reported for total population as well as by toxin type and age group, including young (<6 months) and older (6–12 months) infants, including testing in both ill and well participants;
• population based-surveillance among multiple sites in a country or region to determine the generalizability of data from a specific study site;
• temporal distribution of ETEC over multi-year periods, with reporting using consistent descriptors and contextual environmental information such as rainfall and flooding;
• comprehensive stool analyses, using state-of-the-art diagnostic methods, including isolation of Shigella, Campylobacter, enteropathogenic E. coli (EPEC), Rotavirus, and parasitic infections, in studies of relative frequency of diarrhoeagenic pathogens, including testing in both ill and well participants;
• further characterization of the pathogenicity of different ETEC toxin types and strains in both primary and repeat infections, as well as the impact of infection with multiple pathogens on disease incidence and severity.
Diarrhoea incidence is extremely variable and estimates of pathogen-specific incidence may not be generalizable to populations outside of a specific study site, or to the same study population at a different season, or even the same season in a different year. Estimates of ETEC disease incidence are further complicated by a number of other pathogen-specific factors. The frequency of asymptomatic infection ranges from 0% to 22% [Reference Tin22, 25, 30–36, 40–42, 44, 47, 69–Reference Germani84], and as many as 40% of ill persons with ETEC have other pathogens in their stools, making it difficult to know which pathogen caused the illness [Reference Albert40]. Furthermore, multiple case-control studies have shown that cases are no more likely to have LT-ETEC than controls; therefore, LT-ETEC is thought to less commonly cause ETEC disease or severe ETEC disease than ST-ETEC or LT/ST-ETEC [Reference Qadri48, Reference Clemens60]. ST-ETEC may be more pathogenic because the heat-stable toxin does not cause an immune response, and the pathogenicity of LT-ETEC may be largely limited to first infections of a given strain.
Because of these pathogen-specific factors, ETEC disease incidence estimates from population-based studies may be artificially elevated as they generally do not control for asymptomatic infection or infection with multiple pathogens. At the same time, ETEC is clinically under-recognized as a pathogen, as most clinical laboratories in both resource-poor and resource-rich countries lack the capacity to test for it.
Perhaps the most striking data gap is the absence of population-based data from low-HDI countries, especially in sub-Saharan Africa. These countries may have a high incidence of ETEC infection, and understanding this epidemiology will assist in projecting the impact of pathogen-specific and non-specific preventive measures.
A better understanding of age-specific incidence is also needed; only one study describes the incidence in both young and older infants. Further data may help define age groups at highest risk of infection and complications, as well as the role of breastfeeding in preventing ETEC infection.
In the developing world, the incidence of diarrhoea varies greatly by place, season, and even by year independent of season. Simultaneous studies in multiple sites in one country and studies over multiple years would determine the extent of this variability for ETEC infections, and would improve interpretation of existing data and planning of prevention and control programmes.
Finally, studies of relative frequency of diarrhoeagenic pathogens often do not include identification of Campylobacter, Rotavirus, EPEC or parasites, do not use highly sensitive PCR techniques for the identification of Shigella, and do not present results controlling for asymptomatic infections. Addressing these limitations in future studies will improve our understanding of the relative contribution of different pathogens to disease incidence.
The major data needs regarding mortality and complications from ETEC infections are:
• determination of the economic and social burden due to ETEC infections and mortality;
• calculation of CFRs and mortality rates in population-based studies;
• rates of complications in population-based studies of ETEC infection;
• further studies of frequency of moderate or severe dehydration in adults compared to children and the reasons for any differences;
• studies of long-term complications of ETEC infections, including physical fitness, language ability and cognitive function.
Data on complications and mortality due to ETEC infections is extremely limited, and is generally limited to facility-based studies. Calculation of age-specific case-fatality and complication rates may be achieved by studying a discrete number of health facilities providing care for the majority of a given population, in conjunction with a simultaneous population-based incidence study. The clinical severity of ETEC infections is variable, depending both on the host as well as the bacterium and the specific CFs and toxins present, and such studies may improve the understanding of this variability. Such studies could also be done examining multiple pathogens simultaneously in order to minimize costs and provide data on the relative contribution of different pathogens to serious morbidity and mortality.
Data on long-term complications of ETEC infections is also lacking, and longitudinal cohort studies can be conducted examining the relationship between the frequency and severity of multiple ETEC infections and long-term effects on physical, language and cognitive abilities.
The major data needs regarding preventive measures for ETEC infections are:
• further research into the immunology of natural protection from ETEC, as well as methods of vaccine delivery and optimal vaccine antigens;
• effect of vitamin A and zinc deficiency or supplementation on risk of severe ETEC infections;
• determination of proportion of ETEC infections attributable to different routes of transmission in different settings;
• evaluation of the cost-effectiveness and sustainability of large scale introduction of non-vaccine preventive measures in endemic settings, and the feasibility of rapid introduction of non-vaccine preventive measures in epidemic settings.
Such research is critical to define the most effective prevention measures. Research also needs to continue on cost-effective, sustainable, large-scale models for implementation of more general prevention strategies, such as provision of safe food and water.
The major needs regarding diagnostics for ETEC infections are:
• standardization of the molecular definition of ETEC, based on either PCR or DNA probes for enterotoxins, for surveillance purposes;
• evaluation of the performance of PCR compared to ELISA for toxin identification;
• development of correction factors for other diagnostic methods to enable comparison of incidence results.
Existing data regarding the epidemiology of ETEC infections rests on the quality and consistency of diagnostic methods. In order to be able to compare results from different studies, a standardized molecular definition of ETEC for surveillance purposes is urgently needed. Moreover, because different diagnostic methods are available in different sites, correction factors for different diagnostic methods need to be developed to allow comparison of results to the gold standard. An international microbiological standard, along with a centralized system of laboratory training and quality assurance, would form the foundation for addressing the other data gaps.
Although much is known about the burden of ETEC infections, there are still substantial gaps in knowledge, including large geographic areas and important age groups. Filling in these gaps will require extensive planning and investment, but studies can simultaneously fill in similar gaps for other important infectious causes of diarrhoea. This information is essential in order to direct research into effective interventions, and to target the interventions for maximum impact.
ACKNOWLEDGEMENTS
This work was supported in part by the U.S. National Institutes of Health Fogarty International Center and by grant number 32143 from the Bill and Melinda Gates Foundation ‘Assessment of diarrhoea disease burden and public health programs to control diarrhoea in Asian subcontinent and Africa’.
DECLARATION OF INTEREST
None.









