Introduction
Breast cancer (BC) burden has surpassed lung cancer and was ranked the first diagnosed cancer globally and the first among females in Egypt according to GLOBOCAN 2020 (Refs Reference Sung1, 2). BC molecular classification was grounded on the expression of hormone receptors (HRs) including oestrogen receptor (ER), progesterone receptors (PR), human epidermal growth factor receptors 2 (HER2) and the proliferative index Ki-67 (Ref. Reference Goldhirsch3). Four main molecular BC subtypes have been extensively characterised comprising: luminal A (ER+/PR+/HER2–/lowKi-67) with best prognosis, luminal B (ER+/PR+/HER2–/+/high Ki-67), HER-2 enriched (ER–/PR–/HER2+) and finally the most aggressive triple-negative breast cancer (TNBC) subtype (ER–/PR–/HER2–) which is associated with worst prognosis (Refs Reference Goldhirsch3, Reference Cho4). Inflammation and immune evasion are major key players in BC progression that require urgent consideration (Ref. Reference Jiang and Shapiro5). In 1863, the pathologist Rudolf Virchow noticed the presence of large number of leucocytes infiltrating in tumour tissues and believed that cancer is similar to the process of wound healing in chronic inflammation (Ref. Reference Balkwill and Mantovani6). More than a century later, Dvorak demonstrated that cancer is a wound that does not heal (Ref. Reference Dvorak7). Further studies supported this belief and showed that approximately 25% of all human cancers in adults result from chronic inflammation (Ref. Reference Coussens and Werb8). Treatment with non-steroidal anti-inflammatory drugs such as, celecoxib; a cyclooxygenase-2 selective inhibitor, showed anti-tumour effects in primary BC tissue during clinical trial (Ref. Reference Brandão9). In chronic inflammation, immune cells generate high levels of cytokines in an uncontrolled manner such as tumour necrosis factor-α (TNF-α) that induce accumulation of reactive oxygen and nitrogen species which subsequently interact with DNA leading to permanent genomic alterations, initiation of tumours and malignant cell growth (Ref. Reference Hussain and Harris10). Malignant cells also interact with micro-environment via inflammation, where cancerous cells secrete cytokines, chemokines and transcriptional factors that are molecular players in the regulation of onco-genesis (Refs Reference Dmitrieva11, Reference Hoesel and Schmid12).
It has been a mystery to distinguish between immunogenic and non-immunogenic cell death (ICD) (Ref. Reference Serrano-del Valle13). Many years ago, it was believed that physiologic cell death (apoptosis), which occurs as a cellular byproduct turnover, does not cause any immune response (non-immunogenic) and that apoptotic cells are phagocytosed rapidly without causing inflammation or auto-immunity (Ref. Reference Serrano-del Valle13). In 1994, Polly Matzinger proposed ‘The Danger Model’, which implies that the immune system is more concerned with damage than with foreignness (Refs Reference Matzinger14, Reference Matzinger15). Stressed, injured or dying cells were found to express their fear of danger by releasing mediators called ‘danger-associated molecular patterns’ (DAMPs) that warn the body about tissue injury or danger in these areas and trigger sterile inflammation (Refs Reference Matzinger14, Reference Matzinger15). In healthy cells, DAMPs are kept inside the cell (no inflammatory reaction) (Ref. Reference Matzinger15). On the other hand, when the cells are in danger (stressed or dying), such as cancer cells exposed to radiation or some chemo-therapeutic agents like oxaliplatin or anthracyclines, induction of a specific form of apoptosis named ICD takes place by emission of DAMPs from apoptotic cells (Refs Reference Obeid16, Reference Zitvogel17). Inflammation is an innate defence mechanism (Ref. Reference Amin, Boche and Rakic18). Innate immune cells have pattern recognition receptors (PRRs) that are able to recognise molecules frequently found in microbes or released by injured/necrotic cells through their pathogen-associated molecular patterns (PAMPs) and DAMPs, respectively. Upon detecting DAMPs or PAMPs by PRR, inflammasomes are activated facilitating pyroptosis (a lytic programmed cell death) via induction of caspase 1 activation and the subsequent activation and release of interleukin-1β (IL-1β) and interleukin-18 (IL-18) (Ref. Reference Amin, Boche and Rakic18) (Fig. 1).

Figure 1. Inflammasome complex activation. Pattern recognition receptors sense the presence of PAMPs or DAMPs stimulating the recruitment of ASC/Caspase and their oligomerisation with PRR leading to the formation of inflammasome complex. The in-active pro-inflammatory cytokines (Pro-IL-1β and Pro-IL-18) are then cleaved by caspase into their active forms. PAMPs, pathogen-associated molecular patterns; DAMPs, danger-associated molecular patterns; PRR, pattern recognition receptors; ASC, apoptosis-related speck-like protein containing a CARD; IL-1β, interleukin 1β; IL-18, interleukin 18.
Throughout distinct studies, inflammasomes have been shown to take part in development of several inflammatory disorders like pancreatitis (Ref. Reference Sendler19) and might increase risk of cancer (Ref. Reference Karki, Man and Kanneganti20). In addition, high concentration of pro-inflammatory cytokines IL-1β and IL-18 in tumour tissues was associated with increased carcinogenesis and poor prognosis (Refs Reference Inoue21, Reference Krelin22). In BC, high levels of ATP released from radiotherapy-resistant BC cells promoted inflammasome activation via purinergic receptors leading to increased invasion, angiogenesis and metastasis (Ref. Reference Jin, Shin Ko and Kim23). Nevertheless, literature reported that obesity increased the risk of BC development via increasing leptin and decreasing globular adiponectin, resulting in activated inflammasome and tumour growth (Ref. Reference Raut24). Inhibition of the NLRP3 inflammasome via micro-RNA (miRNA) 223-3p lessened the growth and immunosuppression of human BC in vitro and in vivo (Ref. Reference Zhang25). Activation of AIM2 inflammasome and IL-1β secretion were reported to increase programmed death-ligand 1 (PD-L1) expression and subsequently, suppress anti-tumour immunity (Ref. Reference Su26). Blocking of IL-1β in mouse BC synergises the effect of anti-programmed death-1 (PD-1) and reversed immunosuppression (Ref. Reference Kaplanov27). Tecentriq® (Atezolizumab), the newly FDA-approved anti-PD-L1 antibody, to be used in combination with chemotherapy for the treatment of metastatic TNBC patients (Ref. Reference Marvastzas A28), unfortunately showed modest response rates and was associated with serious immune-mediated adverse events (Ref. Reference Schmid29). Until date, there is no available anti-cancer drug that directly targets inflammasome pathway. This review highlights the effects of inflammasomes pathways in BC and its possible molecular targets, thus sheds the lights on novel strategies in the prevention and treatment of BC.
Literature search was done at the States National Library of Medicine (PubMed). The descriptors used for the search in databases were: ‘inflammasome’ and ‘purinergic receptor’ or ‘P2Y1R’ ‘P2Y2R’ or ‘P2Y4R’ or ‘P2Y6R’ or ‘P2Y11R’ or ‘P2Y12R'or ‘P2Y13R’ or ‘P2Y14R’ or ‘P2X1R’ or ‘P2X2R’ or ‘P2X3R’ or ‘P2X4R’ or ‘P2X5R’ or ‘P2X6R'or ‘P2X7R’ and ‘breast cancer’, ‘adipokine’ or ‘leptin’ or ‘adiponectin’ and ‘inflammasome’ and ‘breast cancer’, ‘none coding RNA’ or ‘miRNA’ or ‘lncRNA’ and ‘inflammasome’ and ‘breast cancer’, ‘inflammasome’ and ‘immune check point’ or ‘PD-1’ or ‘PD-L1’ or ‘CTLA4’ and ‘breast cancer’. Published data, research papers and books were reviewed for their relevance to the aim of the review. The selection was done by reading abstracts first and then reading relevant full-text articles of relevant publications. Criteria for inclusion were: complete, relevant publications, available online, all years were included (no filters), in English, with detailed information about participants, methods and analyses. Criteria for exclusion: duplicate and out of scope publications. Data collection was done during December 2022, and data abstracted was in the form of descriptive information, covering the type of samples used, techniques and findings or effects reported. Bias was limited through the evaluation of the studies through their internal validity rather than the conclusion.
What are inflammasomes?
Inflammasome structure and activation
In 2002, Fabio Martinon was the first to identify the inflammasome complex (Ref. Reference Martinon, Burns and Tschopp30). From the name, it's an inflammatory signalling complex, located inside the cell (Ref. Reference Amin, Boche and Rakic18). The inflammasome is made up of a receptor that works as a sensor (PRR), the adaptor protein apoptosis-related speck-like protein containing a CARD (ASC) and the effector Caspase as reported by Jay Amin et al. (Ref. Reference Amin, Boche and Rakic18). Once PRR senses the presence of DAMPs or PAMPs, oligomerisation of inflammasome components occurs (PRRs and ASC), then ASC polymerises to form helical structure called ASC speck formation, which is essential for recruitment of caspase 1 (Ref. Reference Stutz31), that in turn cleaves and activates IL-1β and IL-18 (Ref. Reference Schroder and Tschopp32). Finally, cell membrane perforations and inflammatory programmed cell death called pyroptosis occur (derived from the Greek terms ‘pyro’, which means fire, and ‘ptosis’, refers to falling) (Ref. Reference Deets and Vance33) (Fig. 1).
PRRs involved in formation of inflammasomes
PRRs recognise distinct ligands and can be classified according to its cellular location into transmembrane and cytoplasmic PRRs (Ref. Reference Amin, Boche and Rakic18). The transmembrane PRRs include Toll-like receptors and the C-type lectin receptors, while cytoplasmic PRRs encompass nucleotide-binding oligomerisation domain-leucine-rich repeats-containing receptors (NLR), retinoic acid-inducible gene-I-like receptors, absent-in-melanoma (AIM)-like receptors and pyrin inflammasome (Refs Reference Amin, Boche and Rakic18, Reference Heilig and Broz34).
ASC and caspases
ASC ‘also called PYCARD’ is responsible for the activation of caspases (Refs Reference Lupfer, Malik and Kanneganti35, Reference Man and Kanneganti36), which are important for association and activation of inflammasome (Refs Reference Man and Kanneganti36, Reference Shi37, Reference Baker38). Caspases are divided into apoptotic (e.g. caspase 8) and inflammatory caspases (e.g. caspases 1, 4 and 5) (Refs Reference Man and Kanneganti36, Reference Shi37, Reference Baker38). According to the type of caspases involved, inflammasomes are divided into the canonical and non-canonical inflammasome (Refs Reference Amin, Boche and Rakic18, Reference Amarante-Mendes39, Reference Kim, Shin and Nahm40). Caspase 1 is activated within canonical inflammasome while caspase 4/5 or 8 is involved in non-canonical inflammasome pathway (Ref. Reference Downs41). Notably, the impacts of canonical and non-canonical inflammasome activation are similar; caspase-1 provokes activation of IL-1β, IL-18 and the release of danger signals, as well as pyroptosis, while caspase-4/5 promotes pyroptosis via cleavage of the pore-forming protein gasdermin D (GSDMD) and triggers a secondary activation of the canonical inflammasome and subsequent cytokine release (Ref. Reference Downs41).
Inflammasome and its inflammatory cytokines in breast cancer
Inflammasome dual impacts in BC
Inflammasome is a double-edged weapon that exhibited dual roles in the modulation of BC tumourigenesis. NLRP3 activation contributed to immune system dysfunction, BC metastasis, invasion and migration (Ref. Reference Hu42). NLR family pyrin domain containing 1 (NLRP1) expressing cells showed upregulated mesenchymal markers (Snail, MMP-9, Vimentin and C-myc), whereas epithelial markers (E-cadherin) were downregulated (Ref. Reference Wei43). Moreover, NLR family CARD domain containing 4 (NLRC4) upregulated vascular endothelial growth factor A (VEGFA), resulting in angiogenesis induction and BC progression (Ref. Reference Kolb44). Elevated NLRP3 levels increased the expression of the proliferative index Ki67 in BC (Ref. Reference Zhang25). In addition, the relative mRNA expression of NLRP3 in BC cell lines (MDA-MB231, MCF-7 and SKBR3) was higher than normal mammary epithelial cells (Ref. Reference Zhang25). Knockdown of NLRP3 in MCF-7 cell lines repressed the expression of Ki67 and decreased immunosuppression (Ref. Reference Hashmi45). NLRP3 was upregulated in TNBC cell lines leading to gemcitabine resistance and enhanced survival of BC cells (Ref. Reference Zheng46). NLRP3 inhibition reduced viability, colony formation and migration of TNBC cells (Ref. Reference Yao47). Literature demonstrated that reactive oxygen species (ROS) can activate inflammasome pathway (Ref. Reference Deng48). Interestingly, TNBC cell lines showed increased ROS levels that prolonged its survival (Ref. Reference Sarmiento-Salinas49). ROS scavenging or repression in TNBC cell lines downregulated NLRP3 leading to inhibition of metastasis (Ref. Reference Wang50), angiogenesis (Refs Reference Tang51, Reference Han52), reduced migration and invasion (Ref. Reference Si53). On the contrary, ROS-activated inflammasome contributed to cell death of MDA-MB231 (Refs Reference Tang51, Reference Zhang54). In addition, AIM2 suppressed proliferation of human BC (Ref. Reference Chen55) and interferon-γ (IFNγ)-induced AIM2 activation promoted apoptosis in MCF-7 BC cells (Ref. Reference Liu, Yi and Liu56). Collectively, the afore-mentioned opposing impacts of inflammasome in BC remain controversial and require further investigation.
IL-1β
IL-1β activation and secretion is primarily dependent on inflammasomes activation. In 1988, North et al. highlighted the ability of IL-1β to exert anti-tumour effects through inducing T-helper-1 (TH1) and T-helper-17 (TH17) responses (Ref. Reference North57). In addition, literature reported that it served as an adjuvant for maturation and expansion of CD4+, CD8+ T cells and promoted adaptive T-cell-mediated immunity (Ref. Reference Ben-Sasson58). However, in BC, the high levels of IL-1β enhanced BC proliferation (Ref. Reference Honma59) and were significantly associated with BC metastasis (Ref. Reference Todorović-Raković60) and invasion (Ref. Reference Paquette, Therriault and Wagner61). Moreover, IL-1β contributed to cisplatin resistance (Ref. Reference Mendoza-Rodríguez62) and doxorubicin resistance in BC cells (Ref. Reference Mendoza-Rodríguez63). In addition, IL-1β induced epithelial mesenchymal transition (EMT) and contributed to methylation of the ER 1 gene promoter. This epigenetic modification led to a significant decrease in ERα levels and promoted BC chemo-resistance (Ref. Reference Jiménez-Garduño64). Interestingly, after neoadjuvant chemotherapy (nCT), literature demonstrated that BC patients might experience change in their tumour subtype leading to adjuvant treatment alteration in 100% of such patients (Refs Reference De La Cruz65, Reference Lim66, Reference Zhao67, Reference Al-Saleh68). That's why HER2 and HR status (including ER and PR) should be evaluated not only before the initiation of nCT but also after nCT (Refs Reference De La Cruz65, Reference Lim66, Reference Zhao67, Reference Al-Saleh68). Since IL-1β contributed to a significant decrease in ERα levels (Ref. Reference Jiménez-Garduño64), further studies should be done to unravel the hidden reasons for such BC subtype conversion post nCT and whether inflammasome pathway and the subsequent IL-1β secretion were responsible for such change. Thus, targeting IL-1β might give promising effects in counteracting ER lowering, chemo-resistance and BC progression.
IL-18
In 1989, IL-18 was first identified as a factor that enhanced IFNγ production from TH1 cells (IL-18 is also known as IFNγ-inducing factor) (Ref. Reference Nakamura69). Similar to IL-1β, it is cleaved and activated by caspase 1. It is produced from a wide range of normal and cancer cell types, where it binds to IL-18 receptor. Its activity is suppressed by the naturally occurring high-affinity IL-18-binding protein that inhibits its binding to IL-18 receptor (Refs Reference Kuppala70, Reference Fabbi, Carbotti and Ferrini71). IL-18 has opposing effects in BC. For instance, intra-peritoneal injection of IL-18 suppressed metastasis of BC to bones (Ref. Reference Nakata72) and canine IL-18 induced apoptosis in BC cells (Ref. Reference Okano and Yamada73). In addition, mesenchymal stem cells suppressed BC proliferation via IL-18 expression in vitro (Ref. Reference Liu74). Contrarily, literature demonstrated IL-18 as a pro-oncogenic cytokine (Ref. Reference Autenshlyus75) where its expression in BC led to enhanced invasion, metastasis (Refs Reference Li76, Reference Bel'skaya, Loginova and Sarf77, Reference Merendino78, Reference Ma and Kong79, Reference Kunts80, Reference El-Deeb, El-Sheredy and Mohammed81), migration (Refs Reference Li76, Reference Yang82), proliferation (Ref. Reference Park83), angiogenesis (Ref. Reference Jung84) and progression (Refs Reference Metwally, El-Mezayen and Ahmed85, Reference Eissa86). Interestingly, polymorphism in IL-18 contributed to BC among postmenopausal women (Ref. Reference Perel'muter87). IL-18-rs1946518, IL-18-607 and IL18-137 gene polymorphisms were significantly correlated with increased risk of BC (Refs Reference Qader88, Reference Xu and Wang89, Reference Li90, Reference Back91). It's also worth mentioning that IL-18 was overexpressed in tumour samples (Refs Reference Zhou92, Reference Srabović93), plasma (Ref. Reference Turchaninova94), saliva (Ref. Reference Bel'skaya, Loginova and Sarf77) and sera (Ref. Reference Metwally, El-Mezayen and Ahmed85) of BC patients compared with control. IL-18 was associated with worst prognosis (Refs Reference Inoue21, Reference Günel95) and its low expression represented better survival (Ref. Reference Wang96). In addition, it contributed to doxorubicin resistance (Ref. Reference Yao97). Combining chemotherapeutics with IL-18-targeted therapy is highly promising since it has been noticed that low levels of plasma IL-18 were predictive of excellent long-term survival in metastatic BC during chemotherapy (Ref. Reference Tiainen98).
Purinergic receptors and inflammasomes in breast cancer
ATP release and purinergic receptors
Purinergic receptors are found in all cell types (Ref. Reference Gicquel99) and are implicated in learning, memory, behaviour, sleep (Ref. Reference Burnstock100), vascular contractility, immune function and growth (Ref. Reference Ralevic and Dunn101). Recently, there is increasing interest in the involvement of purinergic signalling in Corona virus disease 2019 (Covid-19) hyperinflammation, and thrombosis (Ref. Reference Sriram and Insel102). In addition, purinergic receptors were over-expressed in various tumours and regulated proliferation in lung, bladder, prostate tumours (Refs Reference Schäfer103, Reference Shabbir104, Reference Janssens and Boeynaems105) and have been associated with enhanced BC growth and invasion (Ref. Reference Kim106).
Intracellular ATP is produced by mitochondria as a source of energy (Ref. Reference Burnstock107). Damaged cells/tumour cells release ATP outside the cell where it acts as a DAMP (Ref. Reference Burnstock107). In 1999, it was stated that TNF-α, which was highly abundant in tumour micro-environment, has shown to enhance tumour progression (Refs Reference Reed108, Reference Suganuma109) and metastasis (Ref. Reference Egberts110). Literature showed that TNF-α enhanced ATP release, especially in MDA-MB231, and it is radiotherapy resistant (RT-R-MDA-MB231) (Ref. Reference Jin, Shin Ko and Kim23).
The extracellular ATP activates its purinergic receptors (present on adjacent cells) via binding either to the seven P2X ion channel receptors subtypes (P2X 1–7) or the eight known P2Y G-protein coupled receptors subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) (Ref. Reference Burnstock107).
P2Y purinergic receptors in BC
Several studies demonstrated various tumour-promoting effects of P2Y receptors in BC (Refs Reference Duan111, Reference Azimi112, Reference Yokdang113, Reference Yokdang114, Reference Bilbao, Santillán and Boland115, Reference Liu116, Reference Wright117). P2Y1, P2Y6 and P2Y11 inhibition blunted BC metastasis and migration (Refs Reference Duan111, Reference Azimi112, Reference Liu116). In addition, the P2Y1 antagonist MRS2179 lessened BC growth (Ref. Reference Yokdang113) and inhibited angiogenesis via blocking activation of vascular endothelial growth factor receptor 2 (VEGFR-2) (Ref. Reference Yokdang114). The phosphoinositide 3-kinase (PI3K)/protein kinase B (also known as AKT; PI3K/AKT) pathway is a regulator of pivotal cell functions such as cell proliferation and survival (Ref. Reference Cantley118). Literature showed that ATP modulation of P2Y2/4 receptors increased BC proliferation via activation of the PI3K/AKT signalling pathway (Ref. Reference Bilbao, Santillán and Boland115). P2Y12 inhibition with ticagrelor (TIC) reduced spontaneous platelet aggregation/activation in BC patients and inhibited formation of large tumour cell-induced platelet–platelet aggregates (Ref. Reference Wright117).
Expression of P2Y2R and inflammasomes in breast cancer
Findings indicated that P2Y2R expression was higher in tumour tissues of BC patients compared with normal epithelial tissues (Ref. Reference Kim106). In addition, the highly metastatic BC cells (MDA-MB-231) secreted higher ATP and showed elevated P2Y2R activity than low metastatic (MCF-7) BC cells (Refs Reference Jin, Shin Ko and Kim23, Reference Jin119, Reference Joo120) and was associated with tumour progression, invasion and metastasis (Refs Reference Kim106, Reference Jin119, Reference Joo120, Reference Eun121). Interestingly, comparing MDA-MB231, MCF-7 and T47D with their radiotherapy-resistant BC cells (RT-R-MDA-MB231, RT-R-MCF7 and RT-R-T47D) showed that the radiotherapy-resistant BC cells released higher ATP than other BC cells (Ref. Reference Jin, Shin Ko and Kim23); whereas RT-R-MDA-MB231 BC cells showed highest P2Y2R activity and invasiveness (Ref. Reference Jin, Shin Ko and Kim23). Similarly, mRNA levels of NLRP3, NLRC4, ASC, cleaved caspase1 and IL-1β were higher in radiotherapy-resistant BC cells and showed enhanced invasiveness compared to MDA-MB-231 cells (Ref. Reference Jin and Kim122). However, the expression of NLRP1 and AIM2 was lower in RT-R-MDA-MB231 than MDA-MB231 (Ref. Reference Jin and Kim122).
Inflammasome components regulated by P2Y2R activation in breast cancer
Treatment with ATP triggered elevation of P2Y2R activity in MDA-MB231, MCF-7, T47D and their radiotherapy-resistant BC cells (Ref. Reference Jin and Kim122) leading to increased invasiveness (Refs Reference Jin, Shin Ko and Kim23, Reference Jin and Kim122). In MDA-MB231 and RT-R-MDA-MB231 BC cells, TNF-α or ATP treatment led to a significant increase in NLRC4, ASC and IL-1β protein levels in a P2Y2R-dependent manner (Ref. Reference Jin and Kim122). Interestingly, MDA-MB231 and RT-R MDA-MB231 transfected with siRNA P2Y2R or apyrase (hydrolyses extracellular nucleotides) significantly lowered the increased caspase 1 activity, IL-1β and protein expression of NLRC4 and ASC (Ref. Reference Jin and Kim122) that was triggered by TNF-α or ATP in both cells (Ref. Reference Jin, Shin Ko and Kim23).
Inflammasome activation induced invasion and angiogenesis in a P2Y2R-dependent manner in breast cancer
ATP increased the invasiveness in all radiotherapy-resistant BC cells in a P2Y2R-dependent manner where RT-R-MDA-MB231 showed the highest invasiveness (Ref. Reference Jin, Shin Ko and Kim23). Previous literature showed that IL-1β production would increase constantly until late time after stimulation with ATP (Ref. Reference Jin, Shin Ko and Kim23) for many reasons; IL-1β is not only produced by inflammasome activation (Ref. Reference Jin, Shin Ko and Kim23). In 1997, Ferrari et al. showed that ATP-induced IL-1β production (Ref. Reference Korcok123) via activation of nuclear factor-κB (NF-κB) (Ref. Reference Ferrari124) is a critical regulator of fundamental cell functions, such as cell survival and proliferation (Ref. Reference Oida125). In addition, the released IL-1β was found to promote the production of pro-IL-1β by binding to IL-1 receptor, which is expressed in various BC cells including MDA-MB231 (Ref. Reference Pantschenko126). Literature reported that matrix metalloproteinase (MMP) promotes tumour invasion and metastasis by inducing EMT in BC (Ref. Reference Radisky and Radisky127). Furthermore, Yokoo et al. reported that the released IL-1β caused MMP production (Ref. Reference Yokoo and Kitamura128), which was supported further by Amin et al. (Ref. Reference Ruhul Amin129). Similarly, Jin et al. supported these findings, where TNF-α and ATP increased MMP-9 activity in MDA-MB-231 and RT-R-MDA-MB-231 cells (Ref. Reference Jin, Shin Ko and Kim23) (Fig. 2). Similarly, in RT-R-MDA-MB231 and MDA-MB231 BC cells, ATP treatment markedly induced the secretion of VEGFA (Ref. Reference Jin and Kim122), which is known to be produced by hypoxic tumour cells to induce angiogenesis and survival through binding to VEGFR (Refs Reference Ferrara130, Reference Allavena131, Reference Claesson-Welsh and Welsh132, Reference Takahashi133).
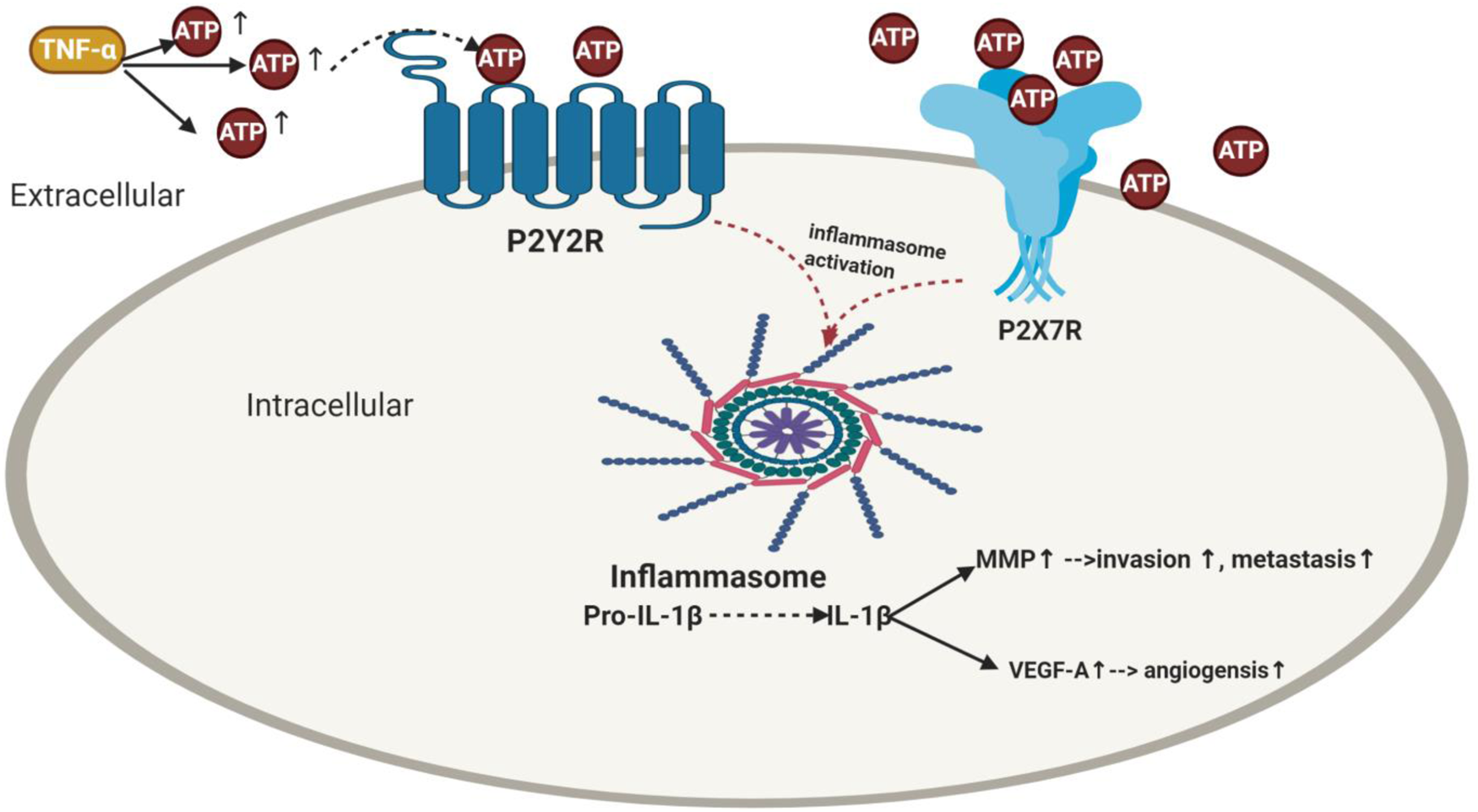
Figure 2. Impact of purinergic receptors on inflammasome activation in breast cancer and its correlation with angiogenesis, invasion and metastasis. TNF-α enhances ATP release. The extracellular ATP activates its purinergic receptors via binding either to the P2X7R receptors or P2Y2R provoking activation of inflammasome as well as IL-1β release which then elevates the levels of MMP and VEGF-A leading to BC metastasis and angiogenesis. TNF-α, tumour necrosis factor-α; ATP, adenosine triphosphate; IL-1β, interleukin-1β; MMP, matrix metalloproteinases; VEGF-A, vascular endothelial growth factor A.
Inflammasome/P2Y2R inhibition lessened invasion, angiogenesis and tumour progression in BC
Accumulating evidence showed treating MDA-MB231 and RT-R-MDA-MB231 BC cells with caspase-1 inhibitor or P2Y2R siRNA abolished the MMP-9 activity (Ref. Reference Jin, Shin Ko and Kim23). Moreover, knockdown of P2Y2R or NLRC4, ASC and caspase-1 by siRNA decreased VEGFA production and suppressed the enhanced ATP-induced invasiveness (Ref. Reference Jin and Kim122). Furthermore, addition of selective irreversible caspase-1 inhibitor (Ac-YVAD-CMK) or siRNAs of NLRC4, ASC and caspase 1 inhibited the invasiveness (Ref. Reference Jin and Kim122) and colony formation (Ref. Reference Jin, Shin Ko and Kim23). In an in vivo P2Y2R-knockdown mouse model (RT-R-MDA-MB231-shRNA), results showed that RT-R-MDA-MB231-shRNA exhibited decreased tumour volume, increased body weight and significantly lowered IL-1β compared to cells transfected with empty vector (Ref. Reference Jin, Shin Ko and Kim23). Nonetheless, cells that transfect with empty vector showed higher MMP-9 compared to the P2Y2R knocked down group. From the above-mentioned, it is clear that inflammasome causes tumour progression in RT-R BC through P2Y2R (Ref. Reference Jin, Shin Ko and Kim23) (Fig. 2).
A wrap-up and insights
NLRP1 has been shown to promote BC cell proliferation, invasion and migration through inducing EMT (Ref. Reference Wei43), but unfortunately, the exact molecular mechanism relating the NLRP1 to EMT was not elucidated. It would be worth to investigate the effect of ATP on P2Y2R or NLRP1 activation as well as MMP levels and its relation to EMT. Moreover, since literature showed that NLRC4 increased secretion of VEGFA and its knockdown decreased VEGFA level (Ref. Reference Jin and Kim122), it would be interesting to examine the impact of P2Y2R or NLRC4 knockdown together with sorafenib (a multi-kinase inhibitor of tumour-cell proliferation and angiogenesis), since NLRC4 knockdown might potentiate sorafenib's anti-angiogenic effect.
In BC, the PI3K/AKT deregulation via mutations in PIK3CA gene or inactivation of the tumour suppressor phosphatase and tensin homologue deleted on chromosome 10 (PTEN) have been common in ER+ and TNBC patients, respectively (Refs Reference Guerrero-Zotano, Mayer and Arteaga134, Reference Papa and Pandolfi135). Distinct studies reported that PI3K/AKT increased levels of MMP (Refs Reference Park136, Reference Chen137). Recently, FDA approved the use of the oral PI3K inhibitor alpelisib (Piqray) in the treatment of HR+ metastatic BC patients with mutated PIK3CA (Ref. Reference Markham138). Literature reported that ATP modulation of P2Y2 and P2Y4 receptors increased BC proliferation via activation of the PI3K/AKT signalling pathway (Ref. Reference Bilbao, Santillán and Boland115). In addition, NLRP3 knocked out mice showed an inhibition of the PI3K/AKT/mTOR pathway (Ref. Reference Marín-Aguilar139). Since literature showed that P2Y2R signalling activated NLRC4 inflammasome in BC (Ref. Reference Jin and Kim122), there is an urgent need to examine the impact of P2Y2R/NLRC4 and P2Y2R/NLRP3 on PI3K/AKT pathway. In addition, the synergistic impact of combining the P2Y2 antagonist (AR-C118925) or PSB-16133 (P2Y4R antagonist) (Ref. Reference Müller, Baqi and Namasivayam140) together with alpelisib should be explored in BC. Mammalian target of rapamycin (mTOR) is a master regulator of intracellular metabolism and immune cell activation (Ref. Reference Chou141) and its sustained activity has been shown to provoke resistance to alpelisib (Ref. Reference Cai142) and endocrine treatment in BC (Ref. Reference Thangavel143). Stimulation of the P2Y12R receptor led to the activation of mTOR via PI3K-AKT in vascular smooth muscle cells (Ref. Reference Pi144) highlighting the possible role of P2Y12R signalling in alpelisib resistance. P2Y12R receptor inhibitors, such as clopidogrel (CDL) and TIC, were recommended in the treatment of acute coronary syndromes (Ref. Reference Fuller and Chavez145). It would be interesting to examine the impact of TIC/CDL in alpelisib-resistant BC patients.
Interestingly, platelet aggregations have been associated with tumour evasion, and studies reported that platelets form aggregates with tumour cells creating a ‘cloak’ that shields the tumour cell from immune detection (Ref. Reference Nieswandt146). In BC, TIC inhibited the formation of large tumour cell-induced platelet–platelet aggregates in advanced metastatic BC patients (Ref. Reference Wright117) and inhibited metastasis (Ref. Reference Gareau147). Moreover, CDL enhanced the toxicity of docetaxel and increased antitumour and/or anti-metastatic action of chemotherapeutics such as cyclophosphamide, 5-fluorouracil and mitoxantrone (Ref. Reference Denslow148), in contrast it decreased the anticancer activity of doxorubicin, cisplatin and tamoxifen (Ref. Reference Denslow148). Inhibitory impact of CDL and TIC on NLRP3 was investigated (Refs Reference Borges-Rodriguez149, Reference Huang150). Oral administration of TIC strongly inhibited NLRP3 activation in peripheral blood mononuclear cells from patients with acute coronary syndrome (Ref. Reference Huang150). In addition, CDL inhibited NLRP3 activation in rats (Ref. Reference Borges-Rodriguez149). Thus, collectively, these findings suggest the multi-strike clinical use of P2Y12R inhibitors in BC patients with cardiovascular diseases.
P2X7R and inflammasomes in breast cancer
P2X expression in breast cancer
Literature reported that the expression of P2X7R was elevated in breast tissue undergoing malignant change (Ref. Reference Slater151), where all epithelial cells in all cases of in situ or invasive lobular or ductal carcinoma showed intense P2X7R while normal epithelium was devoid of the cytolytic P2X7R (Ref. Reference Slater151). Interestingly, invasive epithelial cancer cells showed intense cell surface P2X7R receptors, whereas in situ lobular and ductal cases labelled P2X7R intracellularly (Ref. Reference Slater151).
P2X opposing impacts in BC
Silencing of P2X5 receptors inhibited cell proliferation, metastasis and vimentin (an EMT marker) in MDA-MB-468 BC cells (Ref. Reference Davis152). As for P2X7R, various studies have shown that it was highly expressed in BC tissues rather than normal ones (Refs Reference Slater151, Reference Tan153, Reference Zheng154). P2X7 receptor activation in MDA-MB-435s BC cell line led to increased migration and metastasis (Ref. Reference Jelassi155). ATP released by dying cells activated P2X7R leading to invasive BC phenotype via AKT phosphorylation and NF-κB translocation to the nucleus (Ref. Reference Tafani156); which comprises a family of transcription factors and showed major roles in the development and progression of various cancers (Ref. Reference Dolcet157). Another study came in accordance and showed that high expression of P2X7R in T47D BC cells increased invasion, migration and EMT through AKT phosphorylation (Ref. Reference Xia158). This was further supported; P2X7 receptor downregulated the protein expression of E-cadherin and upregulated the production of MMP-13 via AKT pathway (Ref. Reference Brisson159). Moreover, treatment of P2X7R-positive MDA-MB-435s BC cells with the anthraquinone derivative emodin suppressed invasiveness via antagonising P2X7R in vivo (Ref. Reference Jelassi160). Furthermore, P2X7R inhibition effectively induced apoptosis in MCF-7 BC cells (Ref. Reference Tan153). Contrarily, ATP-P2X7R activation inhibited BC cell migration (Ref. Reference Avanzato161); these conflicting impacts of P2X7R in BC require closer investigation.
P2X/inflammasomes opposing effects in breast cancer
Suppression of P2X7R expression by the natural isoquinoline alkaloid ‘berberine’ inhibited mRNA and protein levels of NLRP3 that consequently decreased cell viability, colony formation and migration of MDA-MB231 cells in a dose-dependent manner (Fig. 2) (Ref. Reference Yao47). The role of P2X7R/NLRP3 in bone cancer pain was investigated; Walker-256 BC cells were injected into the tibia of female rats; P2X7R inhibition suppressed the expression of NF-κB, NLRP3/IL-1β signalling and suppressed bone cancer pain in vivo (Ref. Reference Wu162). On the other hand, Ghiringhelli et al. demonstrated that P2X7R/NLRP3 activation showed anti-tumour effects (Ref. Reference Ghiringhelli163); the study demonstrated that ATP stimulated-P2X7R then triggered NLRP3 activation in dendritic cells (Ref. Reference Ghiringhelli163). Furthermore, chemotherapy was inefficient against tumours with P2X7R (−/−) or NLRP3(−/−) or Caspase-1(−/−) (Ref. Reference Ghiringhelli163). Additionally, BC patients bearing a loss of function allele of P2X7R treated with anthracycline developed metastatic disease more rapidly compared to those carrying the normal allele (Ref. Reference Ghiringhelli163).
Wrap-up and future insights
NF-κB played a critical role in endocrine resistance (Ref. Reference Oida125), promoted human BC proliferation and migration via MMP-9 production in vivo (Ref. Reference Oida125). It is worth mentioning that NF-κB is pivotal in the transcription and priming step of NLRP3 activation (Ref. Reference Lebreton164). On the other hand, NLPR3 also activated NF-κB where its knocking down reduced NF-κB activation in both sterile and microbially induced inflammation (Ref. Reference Kinoshita165). Since P2X7R inhibition suppressed the expression of NF-κB and NLRP3 in rats (Ref. Reference Wu162), it would be interesting to examine the impact of P2X7R/NF-κB/NLRP3 in BC as their concomitant inhibition might be eminent in counteracting BC resistance and progression. ATP-P2X4 signalling mediated NLRP3 inflammasome activation: a novel pathway of diabetic nephropathy (Ref. Reference Chen166). Suramin is an old drug that is still being used to treat the first stage of acute human sleeping sickness (Ref. Reference Wiedemar, Hauser and Mäser167) and has been shown to be an antagonist of ATP at P2X purinergic receptors (Ref. Reference Sahu168). Recent literature reported that it inhibited ATP-induced NLRP3 complex formation, caspase-1 and IL-18 expression in mice mesangial (Ref. Reference Oda169). It would be advantageous to examine the impact of P2X4/NLRP3 in BC. In addition, the potential role of suramin on P2X/NLRP3 inhibition should be investigated in BC.
Simvastatin (SIM), a hypocholesterolemic drug, was shown to inhibit P2X7 receptor, NLRP3 inflammasome, IL-1β and IL-18 in rats (Ref. Reference Menze170). Studies reported that SIM showed cytotoxic effects against MDA-MB231 and MCF-7 BC cell lines (Ref. Reference Rezano171). Recent literature stated that SARS-CoV-2 infection triggered extracellular ATP elevation, P2X7 receptors stimulation and NLRP3 inflammasome hyperactivation causing neurological complications in Covid-19 patients (Ref. Reference Ribeiro172). Thus collectively, SIM might show beneficial impacts in SARS-CoV2-infected BC patients. P2X7R showed opposing effects in cancer. Literature reported that P2X7R showed anti-tumour effects and was required for priming of tumour antigen-specific CD8 + T cells via activation of NLRP3, Caspase 1 and the subsequent release of Il-1β (Ref. Reference Ghiringhelli173). Furthermore, in fibrosarcoma cells, antineoplastic mitoxantrone-treated mice triggered a protective immune response preventing tumour growth, but this protective effect was abolished in P2X7R-deficient mice (Ref. Reference Ghiringhelli173); these conflicting effects require closer investigation. A study demonstrated that released ATP had a biphasic effect on invasion and metastasis of MDA-MB-231 BC cell line; where low ATP doses induced inhibition, while high doses induced promotion (Ref. Reference Zhou174); this might be an explanation for the aforesaid conflicting impacts of P2X receptors in BC. Literature reported that, ATP-high MDA-MB-231 BC cells possessed a dramatic increase in their ability to metastasise in a pre-clinical model in vivo (Ref. Reference Fiorillo175). In addition, metastasis was largely prevented by treatment with an FDA-approved mitochondrial ATP-synthase inhibitor, (bedaquiline) (Ref. Reference Fiorillo175). These results give a hint on investigating the impact of bedaquiline on purinergic receptors/inflammasome/MMP in BC patients and its possible use as an anti-metastatic agent in BC
Inflammasome pathway and immune check points in breast cancer
Under normal physiological conditions after tumour destruction and clearance, immune checkpoints act as ‘brakes’ that are involved in maintaining immune homeostasis and also protect against tissue damage and auto-immunity (Ref. Reference Pardoll176). The expression of immune checkpoint proteins can be dysregulated by tumours as an important immune resistance mechanism that ease tumour evasion from anti-tumour immunity and subsequently leading to cancer progression (Refs Reference Schütz177, Reference Kwa and Adams178). In 2018, Dr James P Allison and Dr Tasuku Honjo were awarded Nobel Prize in Physiology or Medicine for their respective discoveries of the immune checkpoint proteins cytotoxic-T-lymphocyte-associated antigen 4 (CTLA-4) and PD-1 (Refs Reference Ballas179, Reference Esteva180). The PD-1 binds to its ligand; PD-L1 then delivers inhibitory signals to T cells leading to T-cell exhaustion and deactivation (Ref. Reference Katz and Alsharedi181). Interestingly, immune check points inhibition (ICI) by monoclonal antibodies (mAb) have led to a surge in the treatment of solid tumours (Refs Reference Ballas179, Reference Esteva180). However, many patients can acquire resistance and immune-related adverse events (irAE) over time (Refs Reference O'Donnell182, Reference Postow, Callahan and Wolchok183). There is now an urgent need to investigate mechanisms of resistance and irAE. Several studies showed that inflammasome pathway led to immunosuppression (Refs Reference Chen184, Reference Theivanthiran185, Reference Bar186).
Immune check points in BC
PD-1/PD-L1 and CTLA-4 expression in BC
Generally speaking in BC, the expression of PD-L1 has been associated with large tumour size, high proliferation, high-grade, ER-negative status and HER2-positive status (Ref. Reference Sabatier187). In light of the fact that BC is highly heterogeneous, PDL-1/PD-1 expression may vary among different molecular subtypes (Refs Reference Cimino-Mathews188, Reference Kim, Lee and Koo189). Interestingly, several studies reported that PD-L1 expression is more commonly found in the more immunogenic subtypes including TNBC and HER2 positive BC (Refs Reference Cimino-Mathews188, Reference Kim, Lee and Koo189). On the contrary, a study of 1091 BC patients, the expression rate of PD-L1 in luminal A was higher than that of the other BC subtypes (Ref. Reference Tsang190). As for PD-1, its highest expression was higher in the basal-like subtype compared to luminal A with lowest PD-1 expression (Ref. Reference Muenst191). Another study showed that in TNBC, the expression rates of PD-L1 and PD-1 were significantly higher than the expression in other subtypes (Ref. Reference Zhou192). CTLA-4 expression in blood of BC patients was seven folds higher than that of healthy donors (Ref. Reference Mao193). High expression of CTLA-4 was frequently identified in TNBC and HER2+ (Ref. Reference Gu-Trantien194).
Inflammasomes and PD-1/PD-L1 in BC
Literature showed that high peripheral levels of polymorphonuclear myeloid-derived suppressor cells (PMN-MDSC) correlate with increased tumour angiogenesis, higher tumour grade, tumour promotion and T-cell-mediated suppression by blocking the development of an effective antitumour immunity (Ref. Reference Najjar195). A recent study reported that genetic and pharmacologic inhibition of NLRP3 blocked PMN-MDSC accumulation in the lung in response to anti-PD-1 therapy and inhibited metastatic progression in preclinical BC models (Ref. Reference Theivanthiran196). Under hypoxic conditions in MDA-MB231 PD-L1 translocated to the nucleus (nPD-L1) upon TNF-α treatment. nPD-L1 enhanced the transcription of the gasdermin C (GSDMC) and its cleavage by caspase 8 causing non-canonical pyroptosis which was associated with BC poor prognosis in nude mice (Ref. Reference Hou197). Since cleavage of the pore-forming protein GSDMD triggered a secondary activation of the canonical inflammasome (Ref. Reference Downs41), the involvement of PD-L1/GSDMC/canonical inflammasome activation warrants investigation. Another study demonstrated that AIM2 inflammasome upregulated PD-L1 via IL-1β leading to immunosuppression in BC whereas neutralisation of IL-1β significantly suppressed PD-L1 (Ref. Reference Su26) (Fig. 3). Similarly, blocking IL-1β in mouse BC reversed immuno-suppression and synergised the effect of anti PD-1 (Ref. Reference Kaplanov27). In TNBC cells, tumour-derived IL-18 induced PD-1 expression on immunosuppressive NK and in B cells (Refs Reference Park198, Reference Zhao199).
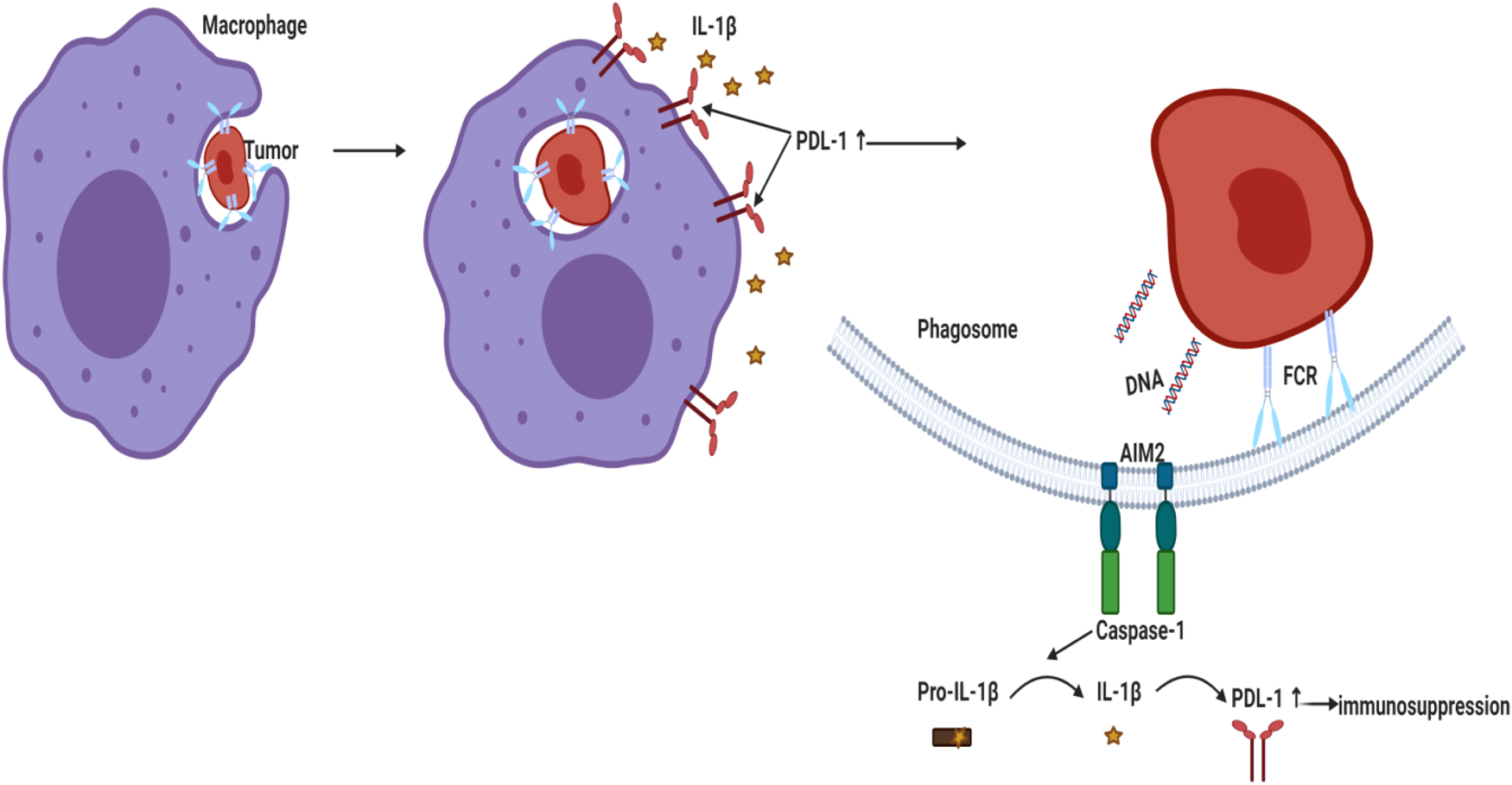
Figure 3. Activation of AIM2 inflammasome led to PD-L1 upregulation and immunosuppression in BC. Phagocytosed tumour DNA activated AIM2 inflammasome leading to IL-1β secretion that upregulated PD-L1 and enhanced immunosuppression. AIM2, absent in melanoma 2; PD-L1, programmed death ligand 1; IL-1β, interleukin-1β.
Inflammasomes and CTLA-4 in BC
Little is known about the impact of inflammasomes on CTLA-4. However, in May 2022, Khandekar et al. demonstrated that BC-bearing mice in high salt diet cohort coupled with anti-CTLA-4 mAb showed upregulated NLRP3 complex activity leading to irAE while downregulated NLRP3 diminished irAE in low salt diet cohort plus anti-CTLA-4 mAb, suggesting the involvement of NLRP3 pathway in irAE which is a major clinical challenge in the treatment with ICIs (Ref. Reference Khandekar200).
Wrap-up and insights
TNF-α led to the stabilisation of PD-L1 via NF-κB in BT549 TNBC cell line (Ref. Reference Lim201). In addition, TNF-α enhanced ATP release in MDA-MB231/RT-R-MDA-MB231 (Ref. Reference Jin, Shin Ko and Kim23) and activated P2Y2R/NLRC4 leading to enhanced invasiveness (Ref. Reference Jin and Kim122). Moreover, TNF-α blockade synergised with anti-PD-1 (Ref. Reference Bertrand202). Thus, there might be a link between the effects of NF-κB/TNF-α/ATP release, P2Y2R/NLRC4 and PD-L1 immunosuppression to be urgently investigated. Also, the effects of apyrase, or P2Y2R antagonist/siRNA or caspase 1 inhibitor and their impacts on PD-1 expression in BC cells are quite promising to be explored. Examining the effects of the above-mentioned players in TNBC cells treated with atezolizumab is also tempting since inhibition of ATP/P2Y2R/NLRC4 pathway might possess a synergistic effect. Thus, development of a new combination therapy, lowering the needed concentration of atezolizumab and consequently, ameliorating irAEs.
Several studies showed that inflammasome pathway led to immunosuppression (Refs Reference Chen184, Reference Theivanthiran185, Reference Bar186). In addition, NLRP3 inflammasome promoted the expression of PD-1 in head and neck squamous cell carcinoma (Ref. Reference Chen184) and PD-L1 in pancreatic cancer (Ref. Reference Li203). Interestingly, not only NLRP3 promoted the expression of PD-1 or PD-L1 but also tumour PD-L1 activated NLRP3 inflammasome leading to resistance in response to PD-1 blockade in advanced melanoma (Ref. Reference Theivanthiran185). Surprisingly, PD-1/or PD-L1 immunotherapy blockade also activated NLRP3 inflammasome that recruited myeloid-derived suppressor cells (MDSCs) causing immunotherapy resistance (Ref. Reference Theivanthiran185). In asymptomatic multiple myeloma, these results were further supported in human DCs where PD-L1 blockade activated NLRP3 and increased caspase-1 (Ref. Reference Bar186) (Fig. 4). Collectively, NLRP3 and PD-1/PD-L1 create an immunosuppressive loop leading to immunotherapy resistance that warrant closer investigation in BC. Activation of AIM2 led to PD-L1 upregulation and immunosuppression in BC (Ref. Reference Su26). In addition, cisplatin increased the expression levels of PD-L1 (Ref. Reference Li204). Thus, it would be interesting to investigate the effect of siRNA AIM2 in combination with cisplatin and PD-1/PD-L1 blockade in BC since it might be a promising solution to immune checkpoint resistance and the tumour-associated immunosuppressive effects.
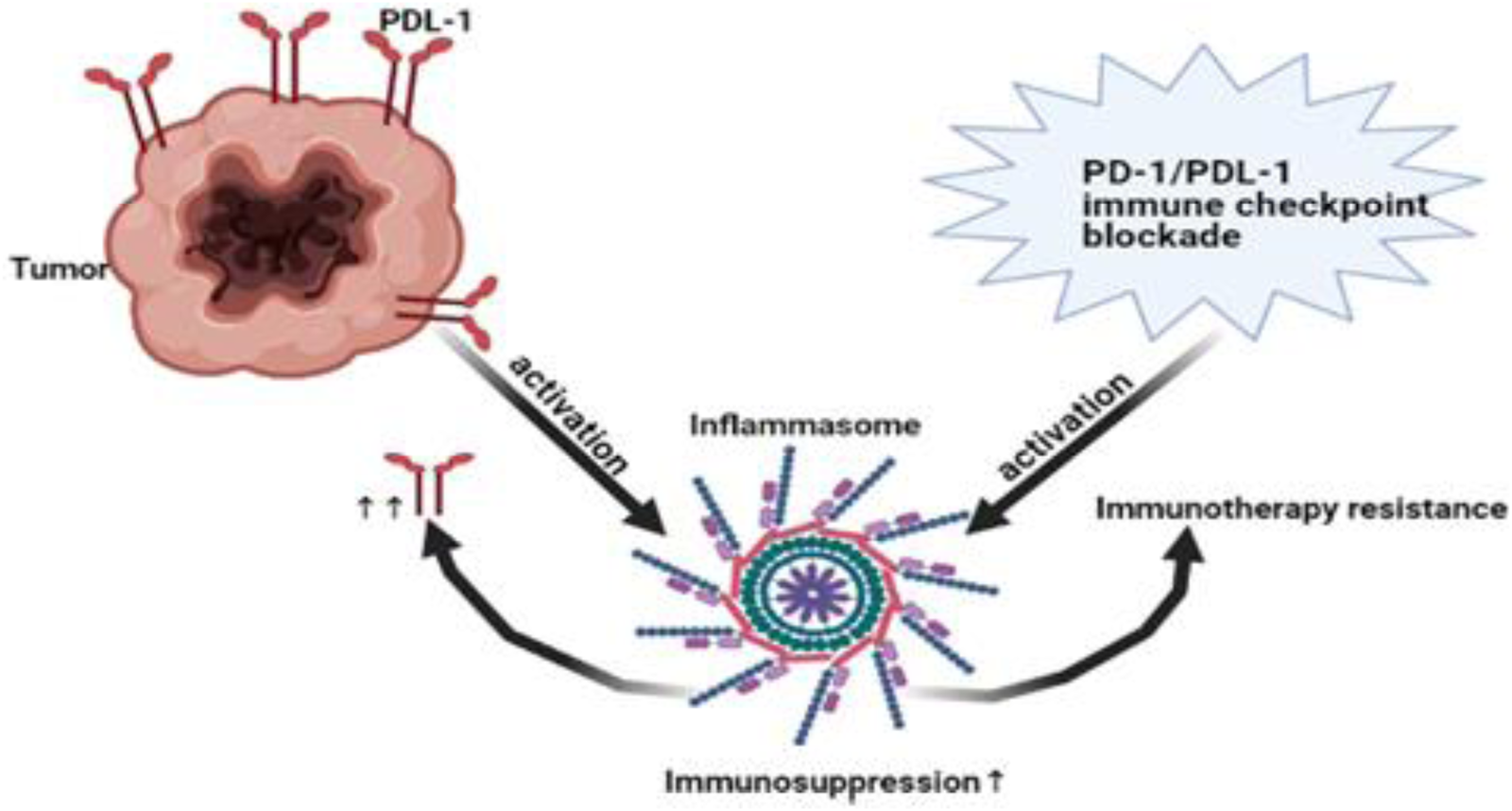
Figure 4. Immunosuppressive loop of NLRP3/PD-L1. NLRP3 promoted immunotherapy resistance via expression of PD-1/PD-L1. On the other hand, tumoural PD-L1 and even PD-1/PD-L1 blockade activated NLRP3 leading to immunotherapy resistance and immunosuppression. Thus, NLRP3 pathway and PD-1/PD-L1 are key characteristic for immunotherapy resistance. PD-1, Programmed death1; PD-L1, programmed death ligand 1; NLRP3, NOD-like receptor (NLR) family, pyrin domain-containing protein 3.
Studies showed that inflammasome activation increased the secretion of IL-1β that subsequently elevated MMP-9 (discussed in purinergic part) (Ref. Reference Jin, Shin Ko and Kim23). Furthermore, MMP-9 inhibition coupled with anti-PD-L1 increased TCR diversity and TH-1 response in tumours (Ref. Reference Juric205). Interestingly, tumour cells not only express PD-L1 on its surface but also can secrete a soluble form of PD-L1 with an immunosuppressive function (Ref. Reference Frigola206) that can be generated by cleavage from cell surface by MMP (Ref. Reference Chen207). In 2020, a study reported that secreted PD-L1 could be used as a non-invasive biomarker for evaluating the malignancy of TNBC and predicting the response to nCT (Ref. Reference Li208). In addition, there was a significant correlation between tumoural PD-L1 and the soluble PD-L1 in the serum of BC patients (Ref. Reference Han209). Furthermore, high levels of soluble PD-L1 in peripheral blood were associated with poor prognosis (Ref. Reference Han209). Hence, it would be tempting to explore the impact of inflammasome/MMP and soluble PD-L1 in BC.
Blocking IL-1β in mouse BC reversed immunosuppression and synergised the effect of anti PD-1 (Ref. Reference Kaplanov27). Similarly, IL-18 induced PD-1 expression in BC (Refs Reference Park198, Reference Zhao199). Thus, targeting inflammasome complex and its downstream cytokines might counteract metastasis and PD-1/PD-L1 immunotherapy resistance. It has been noticed that cells treated with IL-12/15/18 cytokines induced cell surface expression of CTLA-4 on mucosal-associated invariant T independent of TCR signal (Ref. Reference Berkson210). Since NLRP3 activation and irAE occurred post CTLA-4 mAb in BC-bearing cohort (Ref. Reference Khandekar200), NLRP3/IL-18/CTLA-4 and irAE warrant closer investigation in BC.
Adipokines and inflammasomes in breast cancer
Obesity is a risk factor for developing different types of cancers including BC and is associated with a worse clinical outcome (Ref. Reference Reeves211), especially in postmenopausal women (Refs Reference Rose and Vona-Davis212, Reference Colditz and Lindsay213, Reference Kolb, Sutterwala and Zhang214). Obesity symbolises a chronic low-grade inflammatory condition, which causes elevation in the circulating pro-inflammatory cytokines that recruit macrophages into adipose tissue, subsequently leading to dysregulated secretion of adipokines (cytokines derived from adipose tissue) (Ref. Reference Unamuno215).
Adipokines include leptin and adiponectin, their plasma ratio is considered a biomarker for initial cancer development and progression (Ref. Reference Grossmann216). Leptin is principally secreted by adipocytes to suppress appetite, food intake and regulate body weight by acting on hypothalamus (Refs Reference Mechanick, Zhao and Garvey217, Reference Campfield, Smith and Burn218). It is associated with obesity since the higher the adipose tissue mass, the more secreted leptin levels (Refs Reference Mechanick, Zhao and Garvey217, Reference Campfield, Smith and Burn218). On the other hand, adiponectin and adiposity are inversely correlated, where adiponectin levels decrease in obese subjects (Ref. Reference Arita219). Interestingly, adiponectin and leptin elicit opposing effects, where leptin showed pro-inflammatory properties inducing the production of IL-6 and TNF-α (Refs Reference Xiong220, Reference Lee221), while adiponectin is an anti-inflammatory agent (Ref. Reference Fang and Judd222). In addition, adiponectin markedly suppressed mRNA of leptin and its receptor. On the other hand, leptin also markedly downregulated adiponectin receptor 1 (adipoR1) mRNA expression in BC (role of adipoR will be discussed in adiponectin part) (Fig. 5).
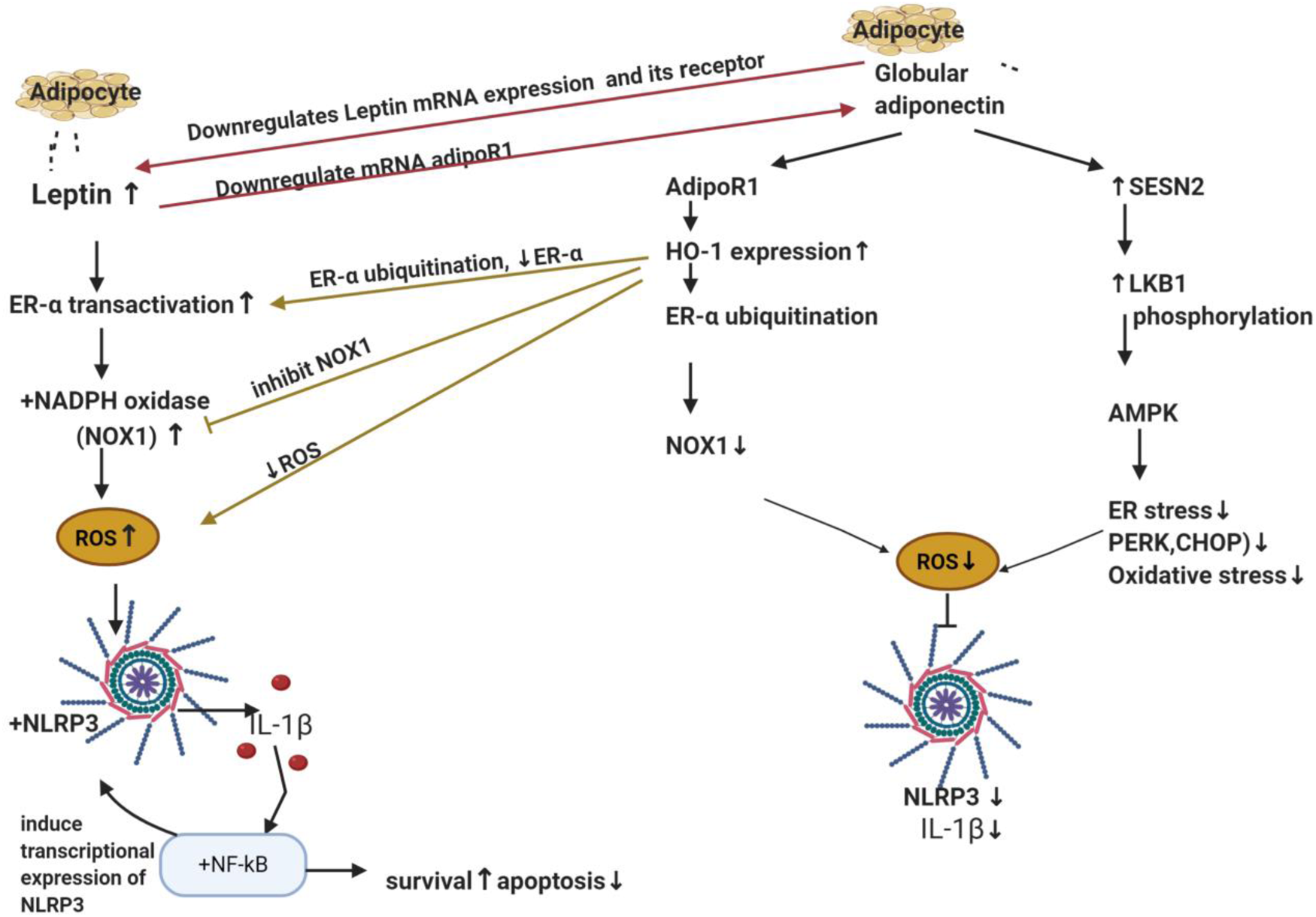
Figure 5. The antagonising effects of leptin and adiponectin on inflammasome in breast cancer. Leptin and adiponectin are adipokines (cytokines) secreted from adipocyte. Leptin transactivates ER-α and activates NADPH oxidase specifically NOX1 which is responsible for ROS production which then activates NLRP3 and IL-1β maturation. The latter activates NF-κB that regulates NLRP3 transcription. Globular adiponectin produces inhibitory actions on NLRP3 and ROS via two mechanisms. The first one through activation of its receptor (AdipoR1), elevation of the anti-oxidant ‘HO-1’and downregulation of ER-α protein expression via ubiquitination and finally lowering the levels of NOX1. The second mechanism is SESN2/LKB1 upregulation and AMPK phosphorylation. It also decreases ER stress markers PERK, its downstream EIF2α and CHOP expression levels. It is worth mentioning that adiponectin and leptin antagonise each other on the transcription level of their receptors; adiponectin suppressed mRNA of leptin's receptor whereas leptin also suppressed adipoR1 mRNA expression in BC. ER-α, oestrogen receptor-α; NOX1, NADPH oxidase 1; ROS, reactive oxygen species; NLRP3, NOD-like receptor (NLR) family; pyrin domain-containing protein 3; IL-1β, interleukin-1β; NF-κB, nuclear factor κ B cells; AdipoR1, adiponectin receptor 1; HO-1, haeme oxygenase 1; SESN2, sestrine2; LKB1, liver kinase B1; AMPK, AMP-activated protein kinase; ER stress, endoplasmic reticulum stress; PERK, protein kinase RNA-like endoplasmic reticulum kinase; CHOP, C/EBP homologous protein.
Collectively, adiponectin and leptin can antagonise each other at the transcriptional level of their receptors or at the level of adipokines production (Ref. Reference Jardé223). Notably, the reduced expression of adiponectin receptors or low plasma levels of adiponectin has been linked to increased risk of certain types of cancers (Refs Reference Katira and Tan224, Reference Dalamaga, Diakopoulos and Mantzoros225). It has been reported that adiponectin exerts anti-tumour activities, while leptin promotes tumour growth (Refs Reference Beales226, Reference Pham227, Reference Illiano228) and it was correlated with hypertension, angiogenesis, atherosclerosis and ROS generation (Ref. Reference Ghantous229). Interestingly, ER signalling plays an important role in proliferation and survival of BC cells through ROS production (Ref. Reference Okoh, Deoraj and Roy230). Leptin activates ER signalling without ligand via a process called transactivation, where leptin activates ERα through mitogen-activated protein kinases pathway (Ref. Reference Catalano231).
Leptin and inflammasomes in breast cancer
Impact of leptin on NLRP3 in ER+/ER– breast cancer subtypes
In ER + BC cells (T47D and MCF-7), leptin upregulated NLRP3, ASC expression as well as ASC speck formation, caspase-1 and IL-1β generation (Ref. Reference Raut24). Literature showed that ER played a pivotal role in survival and proliferation of BC cells through ROS production (Ref. Reference Okoh, Deoraj and Roy230). In MCF-7 BC cells, leptin rapidly increased ROS production that caused NLRP3 activation (Ref. Reference Raut24). Nevertheless, pre-treatment with N-acetyl cysteine (a ROS scavenger) significantly inhibited leptin-induced NLRP3 and ASC overexpression, as well as IL-1β and caspase-1 activation (Ref. Reference Raut24). Furthermore, pre-treatment of MCF-7 cells with tamoxifen (a selective oestrogen receptor modulator) or siRNA against ERα significantly decreased leptin-induced ROS, IL-1β maturation, caspase-1 production, as well as ASC speck formation and returned growth to almost normal levels (Ref. Reference Raut24). On the other hand, treating MCF-7 cells with oestradiol increased leptin-induced growth and IL-1β maturation (Ref. Reference Raut24). NADPH oxidase (NOX) is a membrane-bound enzyme that is responsible for the production of ROS in response to specific physiological stimuli (Ref. Reference Magnani and Mattevi232). NOX is an enzyme complex composed of multiple subunits. In MCF-7 BC cells, NOX1 and NOX2 subunits are mainly expressed (Ref. Reference Juhasz233) where leptin was found to induce NOX activation. Nonetheless, pretreatment with diphenyleneiodonium (a pharmacological NOX inhibitor) inhibited leptin-induced ROS production and reduced leptin-induced increase in NLRP3, caspase 1 activation, ASC expression and speck formation (Ref. Reference Raut24). Notably, leptin did not cause a remarkable change on NOX2 expression; however, it dramatically increased NOX1 mRNA and protein expression in a time- and dose-dependent manner (Ref. Reference Raut24). Adding to that, NOX1 knockdown prevented leptin-induced ROS production and suppressed IL-1β maturation in MCF-7 BC cells. In addition, gene silencing of ERα markedly abolished leptin-induced NOX1 expression (Ref. Reference Raut24). Moreover, oestradiol treatment increased NOX1 expression in MCF 7 cells (Ref. Reference Raut24) (Fig. 5).
The ER-deficient MDA-MB231 BC and SK-BR-2 cells treated with leptin showed no significant effects on NLRP3, ASC or IL-1β protein expression (Ref. Reference Raut24). MDA-MB231 BC cells treated with leptin showed non-significant changes in ROS generation or in IL-1β maturation. In addition, the ER-negative SK-BR-3 cells treated with leptin showed no significant effects on NLRP3 protein expression or on ASC protein expression (Ref. Reference Raut24). Collectively, this proves that ER plays a pivotal role in ROS and inflammasome activation induced by leptin in BC cells.
Impact of leptin-induced NLRP3 activation on apoptosis and cell cycle progression in breast cancer
In BC, leptin increased the number of viable cells, suppressed caspase-7 and pro-apoptotic Bcl-2 associated X-protein (BAX), and increased expression of anti-apoptotic B cell lymphoma 2 (Bcl2) (Ref. Reference Raut24). Thus, it collectively inhibited apoptosis. Furthermore, it induced the expression of cyclin D1 and enhanced cell cycle progression (Ref. Reference Raut24). In BC cells, addition of selective NLRP3 inhibitor or transfection with siRNA against NLRP3 prevented the leptin stimulated tumour growth, restored caspase 7, abolished suppression of BAX and prevented leptin-induced Bcl2 expression. In addition, it also significantly decreased the populations of cells in S and G2-M phase and enhanced cell populations in G0–G1 phase of cell cycle and significantly reduced leptin-induced cyclin D1 expression (Ref. Reference Raut24).
Leptin-induced inflammasome activation via ROS in vivo
Similarly, in MCF-7 tumour xenograft mice, treatment with leptin led to increased tumour growth, volume, size and weight (Ref. Reference Raut24). Leptin also led to increased IL-1β, Bcl2 and cyclin D1 expression, and finally decreased the expression of BAX. Furthermore, treatment with tamoxifen prevented leptin-induced IL-1β, caspase 1 maturation, as well as ASC and NLRP3 inflammasome expression (Ref. Reference Raut24). Moreover, in xenograft model, co-administration of Ac-YVAD-CMK; a capasase-1 inhibitor, with leptin has remarkably decreased leptin-induced tumour growth, IL-1β maturation, Bcl2 and cyclin D1 expression, and significantly restored BAX expression (Ref. Reference Raut24). Collectively, in vivo and in vitro observations showed that obesity increases plasma levels of leptin that transactivates ER receptor leading to activation of NOX, increase in NOX1 expression and ROS generation leading to activation of inflammasome, tumour growth via modulating apoptosis and cell cycle progression (Ref. Reference Raut24).
Wrap-up and insights
It would be beneficial to dig for the exact mechanism by which NLRP3 inflammasome affected the expression of apoptosis-related genes. Inflammasome activation subsequently led to IL-1β maturation and secretion, increased tumour growth and decreased apoptosis (Ref. Reference Raut24). Literature stated that IL-1β activated NF-κB pathway (Ref. Reference Eyre234) that increased survival and decreased apoptosis (Ref. Reference Dolcet157). Recent studies showed that NLRP3 expression can be regulated at the transcriptional level through NF-κB-dependent pathway (Ref. Reference Liu235) (Fig. 5). From all of the above-mentioned, it would be worth investigating the inflammasome/NF-κB pathway in BC and clarifying the exact mechanism of modulating the expression of apoptosis-related genes.
It has been stated that leptin-induced production of TNF-α (Refs Reference Xiong220, Reference Lee221) and TNF-α induced an increase in extracellular ATP (Ref. Reference Jin, Shin Ko and Kim23), which in turn, activated purinergic receptors and led to invasion and metastasis via inflammasome activation (check purinergic part). In addition, literature reported that TNF-α was able to stabilise PD-L1 contributing to immunosuppression (Ref. Reference Lim201). TNF-α blockade synergised with anti-PD-1 (Ref. Reference Bertrand202). Therefore, it is promising to investigate the effects of leptin/TNF-α release and its correlation with PD-L1 immunosuppression. Blocking this pathway in obese ER-positive BC patients might give a promising synergistic effect in combination with anti-PD-1 or anti-PD-L1 immunotherapy. In addition, further investigation of the effect of leptin-induced ATP release and the subsequent purinergic/inflammasome activation, as well as its effect on MMP and VEGFA in BC cells is interesting; since this pathway might highlight targets that might counteract invasion and angiogenesis in BC. Furthermore, leptin showed pro-tumour effects and LDFI (a leptin antagonist) markedly decreased tumour growth in xenograft models, thus LDFI might be very beneficial to investigate its effect if used in combination with chemotherapy, especially in obese patients (Ref. Reference Catalano236). Literature reported that leptin directly stimulated IL-18 expression and promoted migration and invasion of BC cells. Surprisingly, these effects were abolished by the co-incubation of Bay11-7082 (a pharmacological NF-κB inhibitor) (Ref. Reference Li76). Since IL-18 induced PD-1 expression in BC (Refs Reference Park198, Reference Zhao199), it would be worth to investigate the effect of combining Bay11-7082 and leptin on inflammasome/PD-1 expression and whether Bay11-7082 would give a synergistic effect if co-administered with anti-PD-1.
Globular adiponectin
Adiponectin exists as a full-length protein of 30 kDa (Ref. Reference Fang and Sweeney237). It is the most abundant adipokine in the circulation where it accounts for approximately 0.01% of total plasma proteins (Refs Reference Divella238, Reference Rajala and Scherer239). Adiponectin's anticancer activities are mediated through different mechanisms including cell cycle arrest, induction of apoptosis and inhibition of migration/invasion of cancer (Refs Reference Shrestha240, Reference Cui241, Reference Nigro242). In addition to the full-length form, globular adiponectin can be generated through proteolytic cleavage, which is a fragment containing the globular domain of adiponectin. Despite the low circulating concentration of globular adiponectin, it possessed potent diverse physiological activities and inhibited LPS primed inflammasomes activation through autophagy induction and AMPK signalling in macrophages (Refs Reference Waki243, Reference Kim244, Reference Wang245)
Globular adiponectin opposed leptin-induced NLRP3 inflammasome activation and breast cancer growth
In MCF-7 BC cells, globular adiponectin showed suppressive effects on inflammasomes activation, where it significantly decreased the levels of IL-1β, caspase 1, NLRP3, ASC speck formation and ASC (Refs Reference Pham227, Reference Raut and Park246). Similarly, globular adiponectin opposed leptin-induced growth of cancer cells and inhibited the leptin-induced NLRP3 inflammasome activation and suppressed the elevated ASC, caspase-1 and IL-1β. Furthermore, it suppressed leptin viability, restored cell cycle and apoptosis to normal levels (Refs Reference Pham227, Reference Raut and Park246).
Globular adiponectin antagonises leptin-induced breast cancer growth via ER-α ubiquitination and HO-1 induction
A pre-proof literature reported that globular adiponectin exerted its inhibitory action on leptin-induced inflammasome activation via upregulation of haeme oxygenase-1 (HO-1) in a dose- and time-dependent manner (Ref. Reference Raut and Park246); which has been reported to exhibit anti-oxidant activities that reduced ROS (Ref. Reference Villalpando-Rodriguez247). Moreover, addition of SnPP (a pharmacological inhibitor of HO-1) or siRNA HO-1 abrogated the suppressive effects of globular adiponectin on NLRP3 and ASC (Ref. Reference Raut and Park246). The biological effects of adiponectin are initiated by binding with its specific transmembrane receptors; adipoR1 or adipoR2 (Ref. Reference Yamauchi248). In MCF-7 BC cells, SiRNAs of adipoR1 or adipoR2 significantly inhibited the upregulation of HO-1 expression and abolished the suppressive effects of globular adiponectin on inflammasome. Notably, adipoR1 SiRNAs showed higher preventative effect (Ref. Reference Raut and Park246). Furthermore, globular adiponectin significantly attenuated leptin-induced ROS and NOX activation (Ref. Reference Raut and Park246). All these effects were abolished with SnPP or gene silencing of HO-1. Interestingly, globular adiponectin markedly downregulated transcriptional activity of ER-α receptor which was enhanced by leptin. Moreover, results showed that globular adiponectin significantly downregulated protein expression of ER-α receptor through increasing ER-α ubiquitination (this explains why it affected its protein level and not its mRNA) (Ref. Reference Raut and Park246) (Fig. 5). Similar to in vitro effects, globular adiponectin inhibited NLRP3, ASC, enhanced apoptosis, decreased Ki67 and decreased tumour volume in vivo (Ref. Reference Raut and Park246).
Globular adiponectin decreased ER stress markers and viability of ER-positive breast cancer cells via suppression of inflammasomes through SESN2/AMPK/ER stress signalling pathway
Accumulation of unfolded proteins in the ER lumen, occurring during ER stress, is sensed by protein kinase RNA-like endoplasmic reticulum kinase (PERK) that induces the activation of unfolded protein response pathways and phosphorylates its downstream eukaryotic initiation factor 2 alpha (eIF2α) (Refs Reference Verfaillie249, Reference Li250, Reference Rozpedek251). In addition, it subsequently activates the transcription factor C/EBP homologous protein (CHOP); a marker for ER stress that propagates ROS signals, contributing to apoptosis (Refs Reference Verfaillie249, Reference Li250, Reference Rozpedek251). Sestrine 2 (SESN2) is known to provide cytoprotection against ER stress and reduces levels of cellular ROS (Ref. Reference Pasha252). It is one of the critical regulators of 5′ AMP-activated protein kinase (AMPK) activation and was found to decrease ROS and oxidative stress (Ref. Reference Pasha252) via its upstream liver kinase B1 (LKB1), that functions as a tumour suppressor gene (Refs Reference Zulato253, Reference Ren and Shen254). Literature reported that LKB1/AMPK boosted Nrf2 and increased HO-1 expression that further decreased ROS and oxidative stress (Ref. Reference Zimmermann255).
Globular adiponectin decreased ER stress markers PERK, its downstream EIF2α and CHOP expression levels (Ref. Reference Pham227) (Fig. 5). Addition of the classical ER stress inhibitor (TUDCA) resulted in a significantly reduced level of mature IL-1β and caspase 1 in a dose-dependent manner (Ref. Reference Pham227). On the contrary, addition of the pharmacological ER stress inducer tunicamycin markedly increased mature IL-1β and caspase 1 in MCF-7 BC cells (Ref. Reference Pham227). Globular adiponectin treatment induced a marked increase in protein expression of SESN2 and phosphorylation of AMPK and increased complex formation of SESN2/AMPK and SESN2/LKB1 in MCF-7 BC cells (Ref. Reference Pham227). Results showed that SESN2 acted as a scaffold for AMPK and LKB-1 (Ref. Reference Pham227). Treatment with compound C (pharmacological inhibitor of AMPK) or gene silencing of AMPKα and SESN2 abolished the inhibitory effects of globular adiponectin on NLRP3 inflammasome (Ref. Reference Pham227) and restored the ER stress markers (PERK, EIF2 and CHOP). In addition, in MCF-7 cells, transfection with SESN2 siRNA inhibited the globular adiponectin-mediated AMPK phosphorylation and completely abolished LKB1/AMPK complex formation (Ref. Reference Pham227). In ER-positive cells (MCF-7 or T47D), inflammasome activation was observed to contribute to their growth, but not in ER-negative MDA-MB231 cells (Refs Reference Raut24, Reference Pham227). Similarly, pharmacological inhibitors of inflammasome (Ac-YVAD-cmk, MCC950 and interleukin 1 receptor antagonist ‘IL-1Ra’) or globular adiponectin significantly decreased the cell viability of MCF-7 and T47D cells but not MDA-MB231 and upregulated the negative modulators of cell cycle: P27kip and P53, downregulated cyclin D1 and induced cell cycle arrest at G0/G1 phase (Ref. Reference Pham227).
In MCF-7, tumour xenograft model established in BALB/c nude mice globular adiponectin, inhibitor of NLRP3 (MCC950) or IL-1Ra, inhibited tumour growth (Ref. Reference Pham227), suppressed markers of cell proliferation including Ki67 and cyclin D1, increased the expression of p27kip1 and enhanced apoptosis (Ref. Reference Pham227). In addition, globular adiponectin decreased the expression levels of inflammasome components, increased phosphorylation of AMPK, expression of SESN2 but decreased CHOP expression (Ref. Reference Pham227). All of the above results revealed that adiponectin decreased the growth of BC cells via suppression of inflammasomes through SESN2/AMPK/ER stress signalling pathway (Fig. 5).
Wrap-up and insights
Globular adiponectin was shown to decrease ER stress, inhibit inflammasome activation in BC cells (Ref. Reference Pham227) and exert anti-tumour activities (Refs Reference Pham227, Reference Illiano228), and low plasma levels of adiponectin have been linked to increased risk of certain types of cancers (Refs Reference Katira and Tan224, Reference Dalamaga, Diakopoulos and Mantzoros225). In addition, a recent study stated that globular adiponectin exerted its inhibitory effect through AdipoR1 (Ref. Reference Raut and Park246). It is also worth mentioning that AdipoRon (AdipoR agonist that binds to AdipoR1 and AdipoR2) induced apoptosis and decreased proliferation in human ovarian cancer cells; in addition, it inhibited the proliferation of myeloma cells and human osteosarcoma (Refs Reference Sapio256, Reference Ramzan257, Reference Wang258). Therefore, it would be beneficial to investigate the effect of AdipoRon in ER+ BC patients and can be investigated further to be used in combination with chemotherapy as it might be a promising drug that decreases BC growth especially in obese patients.
Interestingly, it has been reported that leptin induced production of TNF-α (Refs Reference Xiong220, Reference Lee221) that led to stabilisation of PD-L1. Literature reported the antagonising effect of adiponectin on leptin mRNA and receptor (Ref. Reference Jardé223). Therefore, AdipoRon's effect on PD-1 and PD-L1 expression should be investigated.
Molecular pathways relating globular adiponectin and leptin in BC should be investigated. It has been noticed that leptin activated NLRP3 inflammasome causing increased BC growth via ER-α activation (Ref. Reference Raut24). On the contrary, globular adiponectin enhanced HO-1 expression and SESN2/LKB1/AMPK complex formation (Refs Reference Pham227, Reference Raut and Park246). Literature reported that AMPK activated Nrf2 that increased the expression of HO-1 (Ref. Reference Zimmermann255). This might explain how globular adiponectin enhanced HO-1 expression and subsequently inhibited inflammasome (since the exact mechanism in cancer cells was unclear) (Ref. Reference Raut and Park246). From all the above-mentioned, the effect of adiponectin-induced AMPK/nrf2/HO-1/ER-α ubiquitination and suppression of leptin-induced inflammasome activation and BC growth should be further investigated.
Non-coding RNA and inflammasomes in breast cancer
Initially, non-coding RNAs (ncRNAs) were viewed as ‘transcriptional noise’ (Ref. Reference Xu259). Later on, studies showed that ncRNAs are regulators of crucial biological processes such as cell proliferation, differentiation and invasion (Refs Reference Panoutsopoulou, Avgeris and Scorilas260, Reference Klinge261). According to their length, ncRNAs are divided into long non-coding RNAs (lncRNAs) and short non-coding RNAs (Ref. Reference Xu259). The latter comprises small interfering RNAs, small nucleolar RNAs, microRNAs (miRNAs) and PIWI-interacting RNAs (Ref. Reference Xu259). miRNA and lncRNA represent the most-studied family classes and their deregulation was correlated with BC (Ref. Reference Panoutsopoulou, Avgeris and Scorilas260).
MiRNA and inflammasomes in breast cancer
MiRNAs are 17–25 nucleotides in length and can function as tumour suppressor miRNAs or as oncogenes (oncomiRs) (Ref. Reference O'Brien262). For instance, in the aggressive TNBC, miR-21 and miR-221 significantly overexpressed while miR-205, miR-145 and miR-122a were downregulated (Ref. Reference Venkatesh263). In addition, miRNA-107 was associated with BC progression (Ref. Reference Li264).
MiRNA-223 has been shown to dampen neutrophilic inflammation via NF-κB suppression (Ref. Reference Zhou265). In BC, the only investigated miRNA inhibiting inflammasomes, till date, is miR 223-3p. Intended overexpression of miR-223-3p in human BC cells attenuated the NLRP3 over-expression (wild type), decreased protein expression levels of ASC, IL-1β and IL-18 (Ref. Reference Zhang25), while increased protein expression of IL-10 (Ref. Reference Zhang25). Furthermore, it lowered tumour volume, increased survival and apoptotic rate. In addition, it decreased the number of ki67 and VEGF-positive cells compared to negative control group. Snail gene is a well-known inducer of EMT and has been associated with BC poor prognosis and metastasis via increasing the expression of MMP-9 (Ref. Reference Wu and Zhou266). In the light of the fact that NLRP3 showed opposing impacts in cancer (Ref. Reference Cheng267), a recent study in August 2022 showed that overexpression of snail-regulated miRNA-21 significantly suppressed cisplatin-induced NLRRP3 activation of TAMs leading to chemo-resistance in murine 4T1 BC cells. In addition, miR-21 has been shown to suppress PTEN causing NLRP3 inactivation (Ref. Reference Cheng267).
LncRNA and inflammasomes in breast cancer
LncRNAs are more than 200 nucleotides in length and their dysregulation was associated with BC (Ref. Reference Lv268), for example, the lncRNA BCRT1 was significantly upregulated in BC tissues and was correlated with BC poor prognosis (Ref. Reference Liang269). A recent study provided a theoretical reference and reported eight pyroptosis-related lncRNAs in BC model including AC004585.1, DLGAP1-AS1, TNFRSF14-AS1, AL606834.2, Z68871.1, AC009119.1, LINC01871 and AL136368.1. Furthermore, mRNAs of AIM2, CASP1, CASP4, IL-18 and NLRP1 were co-expressed with AL606834.2, AC004585.1 and LINC01871 (Ref. Reference Lv268). The aforesaid lncRNAs require practical investigation.
A wrap-up and insights
It would be very beneficial to investigate the effect of combining miR-223-3p synthetic nucleotides with chemotherapy in BC cells (luminal B) that have high ki67, since miR-223-3p inhibited NLRP3 and decreased the expression of ki67 and proliferation (Ref. Reference Zhang25). In addition, miR223-3p might give a synergistic effect if used with sorafenib, since it decreased the expression of VEGF in BC (Ref. Reference Zhang25). Literature showed that IL-18 caused immunosuppression by inducing PD-1 expression in cancer (Ref. Reference Terme270), and since miR223-3p overexpression decreased IL-18 (Ref. Reference Zhang25), then it might be a promising adjuvant with anti-PD-1/PD-L1 blockade immunotherapy ameliorating immunotherapy resistance in BC. Moreover, further investigation should be done on ADAMTS9-AS2's effect on miR-223-3p in BC. Most importantly, more miRNAs targeting inflammasome should be investigated in BC, for example, miR-144 that has been extensively studied and was reported to regulate BC invasion, migration and proliferation (Refs Reference Yin271, Reference Pan272). In addition, it was involved in the regulation of radiotherapy sensitivity (Ref. Reference Yu273). Thus, miR-144 is a worth examining candidate to unravel if it can impact inflammasome pathway in BC cells and correlate its expression with ATP/purinergic receptors, especially in ER-positive BC cells.
In a recent study reported by Ren et al. (Ref. Reference Ren274), lncRNA ADAMTS9-AS2 sponged and inhibited miR-223-3p leading to increased NLRP3 expression and triggered pyroptotic cell death in cisplatin-treated gastric cancer cells. These effects were reversed by miR-223-3p overexpression (Ref. Reference Ren274). LncRNA XIST has been extensively studied by our research group (Refs Reference Samir, Salama and El-Tayebi275, Reference Salama, Adbeltawab and El Tayebi276). LncRNA XIST acted as a tumour suppressor where it was downregulated in BC tissues (Ref. Reference Samir, Salama and El-Tayebi275) and was able to suppress PD-L1 expression in MDA-MB231 cells (Refs Reference Samir, Salama and El-Tayebi275, Reference Salama, Adbeltawab and El Tayebi276). In addition, PD-L1-overexpressing BC patients as well as TNBC cell lines showed an inverse correlation with low levels of XIST, where XIST was described as non-invasive cancer immune biomarker for anti-PD-L1 personalised therapy (Ref. Reference Salama, Adbeltawab and El Tayebi276). Surprisingly, downregulation of lncRNA XIST activated NLRP3 inflammasome and increased caspase1, IL-1β and IL-18 in lung cancer cells (Ref. Reference Liu277). Furthermore, overexpression of lncRNA GAS5 induced a time-dependent activation of ASC, caspase-1 and IL-1β in ovarian cancer (Ref. Reference Li278). In mouse macrophages, the lncRNA NEAT1 promoted pyroptosis and enhanced assembly of several inflammasomes (NLRP3, NLRC4 and AIM2) and subsequently increased caspase 1 and IL-1β (Ref. Reference Zhang279). Another study reported that NEAT1 increased the expression of NLRP3 via targeting miR3076-3p (Ref. Reference Zhang280). Moreover, knockdown of lncRNA Gm4419 ameliorated inflammation in diabetic nephropathy through NF-κB/NLRP3 inflammasome (Ref. Reference Yi281). In addition, Gm4419 led to an increase in the transcription of TNF-α, IL-1β and IL-6 through NF-κB (Ref. Reference Wen, Yu and Fu282). Thus collectively, it would be worth investigating the effects of the above-mentioned lncRNAs on inflammasomes in BC, and its correlation with BC progression and immunotherapy resistance.
Conclusion
In spite of dramatic advances in BC diagnosis, personalised treatments and recently approving immunotherapy against aggressive BC unfortunately, therapy failure occurs in many BC patients because of resistance, tumour recurrence and serious side effects. Unluckily, many BC patients were obliged to discontinue the newly approved immunotherapy treatment due to serious irAE and low response rates. There is an urge to dig for new molecular targets to lessen these obstacles and improve overall therapy response. In this review, we have hoped to shed the light on the inflammasome pathway in BC as it might unravel the hidden molecular contributors to therapy failure, tumour progression and immune dysfunction. Additionally, this review highlights several novel molecules targeting the inflammasome pathway, thereby ameliorating tumour progression, therapy resistance and potentiating the effect of immunotherapy that is recently approved in the treatment of BC. It has been shown throughout numerous studies that inflammasomes and the subsequent IL-1β and IL-18 enhanced invasion, metastasis and angiogenesis. Second, it increased proliferation, decreased apoptosis and increased viability of BC, thereby promoted tumour progression and therapy resistance. Additionally, inflammasomes led to immune system dysfunction, increased PD-L1 and PD-1 expression in BC. Figure 6 summarises the tumour-promoting effects of inflammasomes in BC. Finally, there is an urge to target inflammasome pathway in clinical trials against BC, since studies showed that counteracting this pathway synergised PD-L1 blockade, decreased chemotherapy resistance, angiogenesis and tumour growth of BC. Altogether, inflammasome targeting is a promising molecular multi-strike that might win the battle against BC.
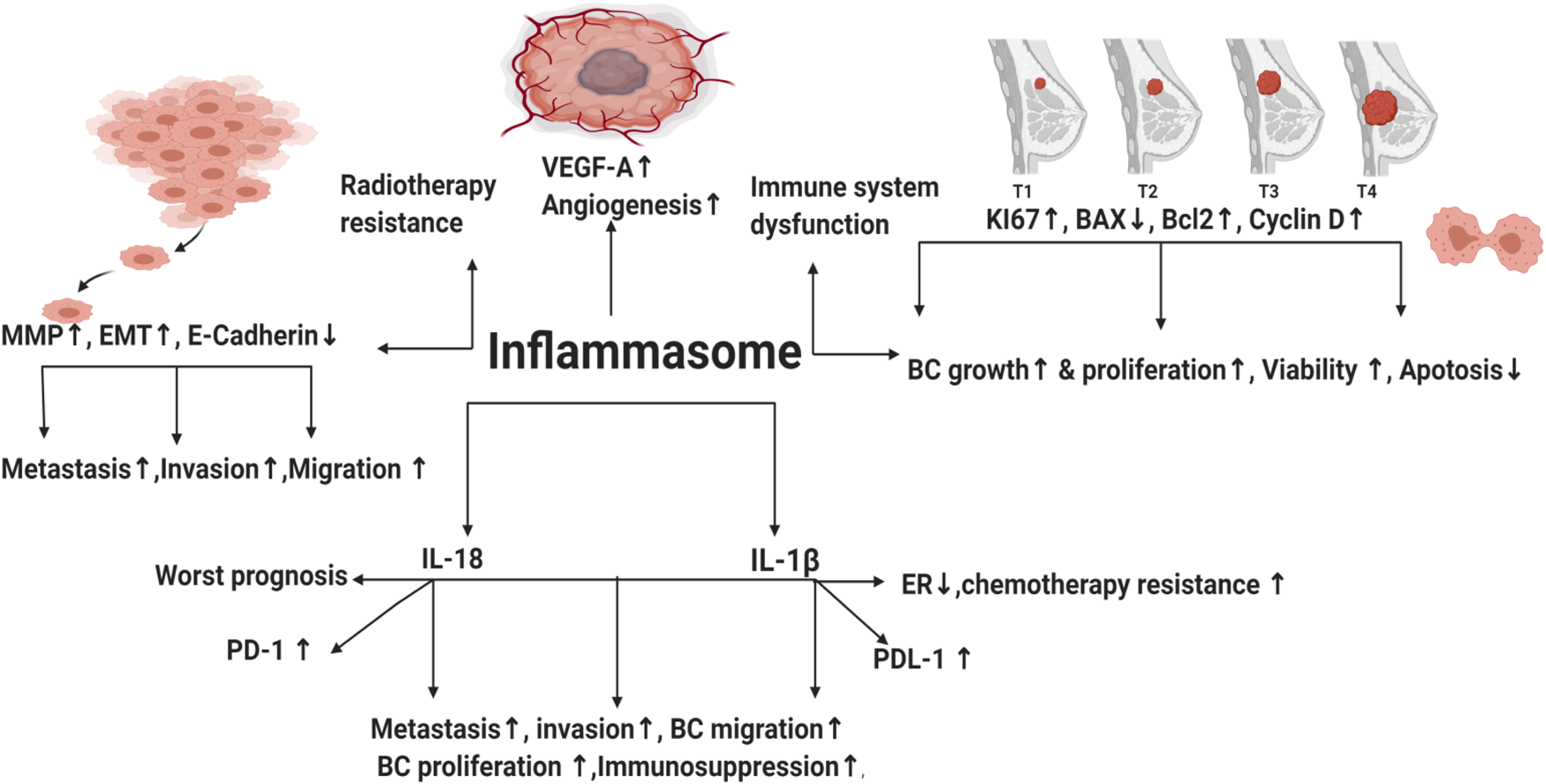
Figure 6. Tumour-promoting effects of inflammasomes in BC. Inflammasomes contributed to therapy failure in BC since it enhanced radiotherapy, chemotherapy and immunotherapy resistance. It led to immune system dysfunction and increased expression of PD-1/PD-L1. In addition, it enhanced cell cycle progression, BC growth and viability through increasing Cyclin D, KI67 and anti-apoptotic BCl2 while lowering pro-apoptotic BAX. Invasion and angiogenic markers such as MMP, EMT and VEGF-A were increased by inflammasomes leading to BC metastasis, invasion and angiogenesis, respectively. Thus, collectively inflammasomes inhibition is a promising multi-strike in BC. IL-1β, interleukin-1β; IL-18, interleukin 18; MMP, matrix metalloproteinases; VEGF-A, vascular endothelial growth factor A; EMT, epithelial mesenchymal transition; ER, oestrogen receptor; BC, breast cancer; KI67, proliferative index; BAX, Bcl-2 associated X-protein; Bcl2, B-cell lymphoma 2.
Competing interest
None.









