INTRODUCTION
Since October 2004, between 35 (2011–2012 season) and 282 (2009–2010 season) influenza-associated paediatric deaths per season have been reported to the Influenza-Associated Paediatric Mortality surveillance system [1]. However, these reports, which include only those deaths for which influenza virus is confirmed by a diagnostic test, probably underestimate the true burden of influenza-associated mortality in children because of factors such as incomplete access to medical care, diagnostic testing practices, and imperfect assay sensitivity [Reference Reed2]. Reports from deaths certificates are an additional system for monitoring influenza-associated deaths; however, these may also underestimate influenza-associated mortality, as some deaths in children that are attributable to influenza may not be recorded as influenza on the death certificate [Reference Hoefer3].
Prior attempts to estimate influenza-attributable mortality have used regression models that fit mortality data to a seasonal baseline function and to indicators of influenza activity from syndromic and/or virological surveillance data [Reference Eickhoff, Sherman and Serfling4–Reference Thompson8]. Any deaths that exceed the baseline function in the fitted model are attributed to influenza. However, there are challenges in applying these methods to estimate excess influenza mortality in children.
Overall, deaths of any cause in persons aged 0–19 years comprise only 2% of total deaths in the United States [9]. Several models of influenza mortality have been applied to children, but few have been constructed specifically to estimate mortality in children. Prior models have limited estimates to underlying respiratory and circulatory deaths because they are expected to be more specific than estimates using all-cause deaths [Reference Thompson8]. While underlying respiratory and circulatory deaths may capture the majority of deaths attributable to influenza in adults, this may not be true for children. The underlying cause of death that is recorded for paediatric fatalities with laboratory-confirmed influenza is often not influenza [Reference Hoefer3]. Additionally, the most frequently reported comorbid condition in children in the United States with influenza-associated death is not respiratory or circulatory disease, but rather neurological disorders [Reference Blanton10–Reference Wong12]. It is likely that limiting a model to respiratory and circulatory deaths in children will miss many influenza-attributable deaths.
In the United States, paediatric deaths associated with influenza are measured not only through death certificate reporting, but also through the Influenza-Associated Paediatric Mortality surveillance system, which collects reports of any laboratory-confirmed influenza-associated death occurring in a US resident aged <18 years [1]. However, prior analyses have not used data on known influenza deaths to refine estimates of influenza-attributable mortality, and some analyses have estimated fewer paediatric deaths in some seasons than are known to occur through laboratory-confirmed reports [1, 13].
The proportion of medical visits for influenza-like illness (ILI) and the proportion of respiratory viral isolates testing positive for influenza virus have both been used as indicators for influenza activity in prior models [Reference Goldstein6, Reference Thompson8, 13]. However, these are not limited to paediatric subjects and may not be appropriate surrogates for influenza mortality in children. Instead of applying all-age surveillance data to children, we use an approach similar to some models [Reference Foppa and Hossain5, Reference Schanzer14, Reference Sprenger15] that use known influenza deaths to predict the total influenza-attributable mortality burden.
METHODS
Mortality data and outcomes
We examined US weekly multiple cause-of-death mortality data for persons aged <18 years from 28 September 2003 to 2 October 2010, using the International Classification of Diseases, 10th Revision (ICD-10) system for classifying causes of death. We included deaths where the underlying cause of death was recorded in one of the following categories, and we excluded deaths where bronchiolitis was an underlying or contributing cause to help exclude deaths attributable to respiratory syncytial virus (RSV). These categories are hereafter referred to as RCNplus: respiratory (ICD-10 codes J00–J99) excluding bronchiolitis (ICD10 code J20), circulatory (ICD-10 codes I00–I99), neurological (G00–G99), sepsis (A40–A41), and other/unspecified viral disease (A81, A85·0–A85·1, A85·8, A86–A89, B01, B04, B07–B09, B25–B34) (Fig. 1). These death categories were chosen because they were commonly recorded as underlying or contributing causes of death for paediatric influenza-certified deaths; i.e. deaths where influenza (ICD-10 codes J09–J11) was reported as an underlying or contributing cause of death. The total influenza-attributable deaths are the influenza-certified deaths plus the additional deaths believed to be attributable to influenza but not certified as such.
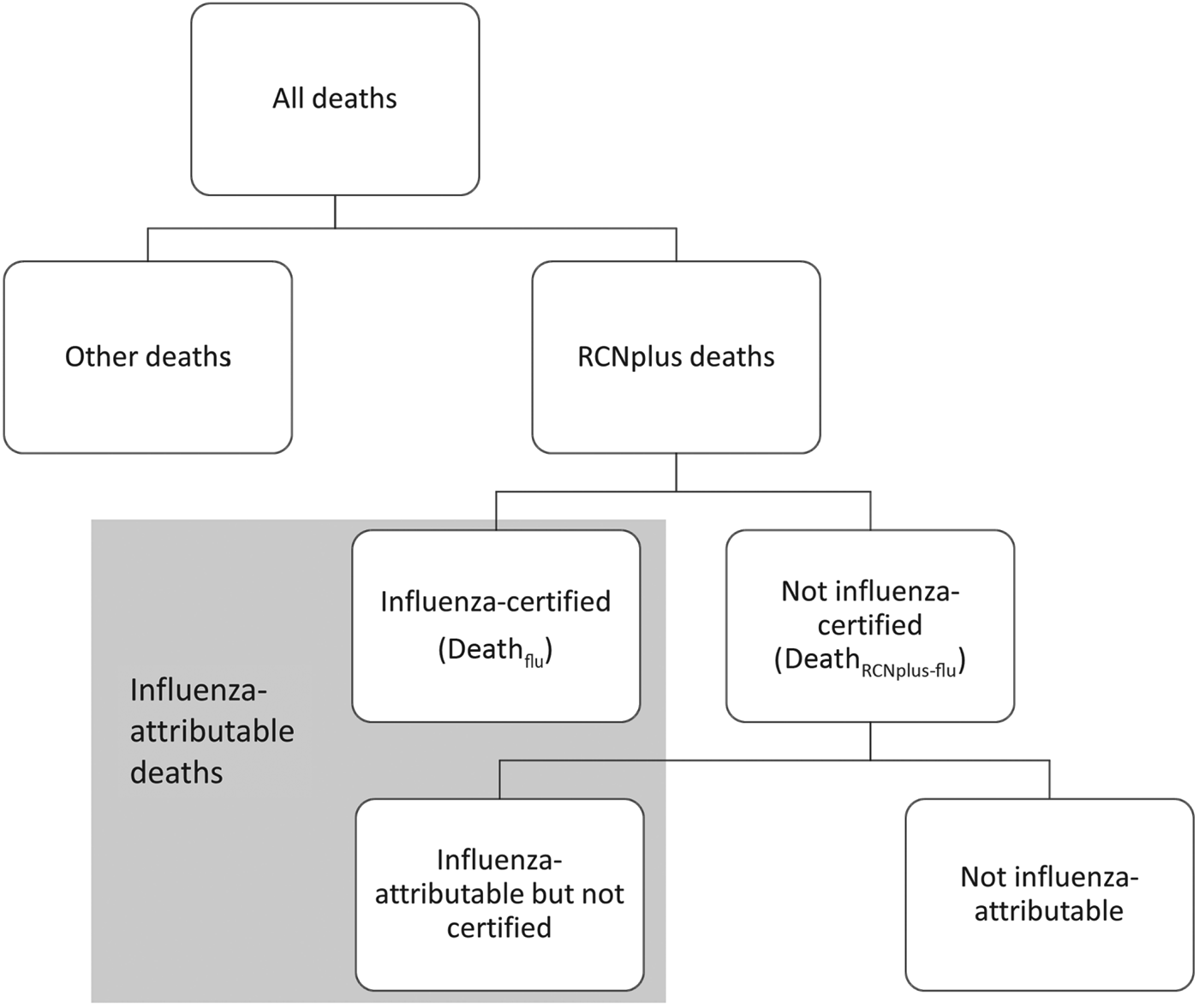
Fig. 1. Categorization of paediatric deaths. RCNplus deaths are those with respiratory, circulatory, neurological, sepsis, or other/unspecified viral illness as underlying cause of death; influenza-certified deaths are those where influenza is listed as an underlying or contributing cause of death. Deathflu includes those during the pandemic period (DeathfluP) and non-pandemic seasons (DeathfluNP).
Definition of influenza seasons and pandemic period
The 2003–2004 to 2007–2008 seasons were defined from week 40 to week 39 of the following year. The 2008–2009 season was defined from week 40, 2008, to week 16 (ending 25 April), 2009. The pandemic period was defined as week 17 (starting 26 April), 2009, to week 39, 2010.
Spline smoothing of influenza-certified deaths
A smoothing spline with 1 knot approximately every 4 weeks was fit to the weekly number of influenza-certified deaths occurring in RCNplus. The smoothed weekly estimates were specified as occurring during the pandemic period (DeathfluP), or outside of the pandemic period (DeathfluNP), to account for potential differences in death certificate-coding practices during that time.
Statistical model
We used a generalized linear regression model to estimate the weekly number of influenza-attributable deaths in children. We included weekly seasonal covariates similar to previous models [Reference Thompson8] and weekly estimates of influenza-certified deaths for the pandemic and non-pandemic periods (Deathflu) within the RCNplus categories. The model was fit to the weekly number of RCNplus deaths minus the RCNplus influenza-certified deaths (DeathRCNplus-flu). DeathRCNplus-flu was assumed to be distributed according to a negative binomial distribution. An identity link function was used because we assumed that non-certified influenza-attributable deaths were proportional to Deathflu. The model was specified as follows:
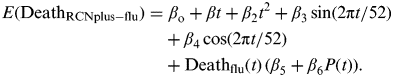 $$\eqalign{E({\rm Death}_{{\rm RCNplus} - {\rm flu}} ) = & \ {\it \beta} _{\rm o} + {\it \beta} t + {\it \beta} _2 t^2 + {\it \beta} _3 \sin (2{\rm \pi} t/52) \cr & + {\it \beta} _4 \cos (2{\rm \pi} t/52) \cr & + {\rm Death}_{{\rm flu}} (t)\,({\it \beta} _5 + {\it \beta} _6 P(t)).} $$
$$\eqalign{E({\rm Death}_{{\rm RCNplus} - {\rm flu}} ) = & \ {\it \beta} _{\rm o} + {\it \beta} t + {\it \beta} _2 t^2 + {\it \beta} _3 \sin (2{\rm \pi} t/52) \cr & + {\it \beta} _4 \cos (2{\rm \pi} t/52) \cr & + {\rm Death}_{{\rm flu}} (t)\,({\it \beta} _5 + {\it \beta} _6 P(t)).} $$
where β 0 is the intercept, t is the week number from 1 to 313, and P(t) = 1 if week t falls into the pandemic period and 0 if t falls into the non-pandemic period. The model was fit to DeathRCNplus−flu for ages 0–17 years. To explore possible differences in causes of RCNplus mortality, including RSV, by age, additional analyses were conducted with DeathRCNplus−flu restricted to ages <2 and ⩾2 years. Smoothing splines were created using proc transreg, and the model was fit using proc genmod (SAS v. 9.2, USA). P < 0·001 was considered statistically significant, and likelihood ratio-based confidence intervals (CIs) are reported. The number of influenza-attributable deaths that were not influenza-certified is β 5[DeathfluNP] + β 6[DeathfluP]. The total influenza-attributable deaths in the RCNplus categories was the sum of these estimated influenza-attributable deaths and the observed influenza-certified deaths, an approach that has been used previously to estimate influenza-associated hospitalizations [Reference Zhou16].
RESULTS
Over the study period, there were 312 268 paediatric deaths of all causes, of which 32 783 (11%) were RCNplus deaths. The RCNplus deaths included 9222 (28%) respiratory, 9623 (29%) circulatory, 9561 (29%) neurological, 3167 (10%) sepsis, and 1210 (4%) other/unspecified viral disease deaths. There were 16 231 (50%) RCNplus deaths in infants aged <2 years and 16 552 (50%) in children ⩾2 years old. Of the RCNplus deaths, 853 (3%) were influenza-certified; 325 (38%) occurred during the pandemic period, and 528 (62%) occurred in the six seasons before the pandemic period.
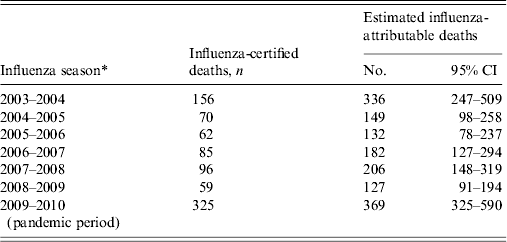
The model fit the predicted outcome, non-influenza certified RCNplus deaths (DeathRCNplus-flu), closely to the observed deaths (Fig. 2). The deviance of the model was 364·6 with 359 degrees of freedom (P = 0·41) and the Pearson χ 2 was 362·2 (P = 0·44). The estimated number of influenza-attributable deaths over the study period was 1501 (95% CI 1113–2402), which is 1·8 (95% CI 1·3–2·8) times higher than the number of influenza-certified deaths over the same period. There were an estimated average influenza-attributable deaths of 214 (95% CI 159–343) per influenza season. In the six seasons before the pandemic period, the number of influenza-attributable deaths was 1132, which is 2·1 (95% CI 1·5–3·4) times higher than the number of influenza-certified deaths during that period. This ranged from 127 estimated deaths during the shortened 2008–2009 season to 336 estimated deaths during the 2003–2004 season (Table 1). During the pandemic period, there were 369 estimated influenza-attributable deaths; this was 1·1 (95% CI 1·0–1·8) times higher than the influenza-certified deaths, although this increase was not statistically significant.

Fig. 2. Observed and predicted influenza-attributable, non-influenza certified deaths (DeathRCNplus−flu) and influenza-certified deaths, United States, 2003–2010. DeathRCNplus−flu, Paediatric deaths in RCNplus (respiratory, circulatory, neurological, sepsis, other viral) excluding influenza-certified deaths; pandemic period: week 17 (starting April 26), 2009, to week 39, 2010.
Table 1. Paediatric influenza-certified and estimated influenza-attributable deaths by influenza season, United States, 2003–2004 to 2009–2010
CI, Confidence interval.
* Influenza seasons defined as week 40 to week 39 of the following year, with the exception of the 2008–2009 season (week 40, 2008 to week 16, 2009), and the pandemic period (week 17, 2009 to week 39, 2010).
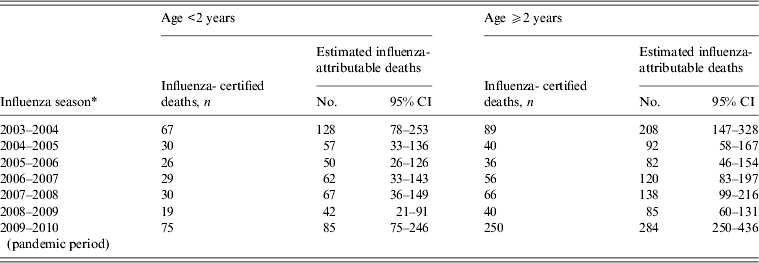
In the models restricted by age group, there were 489 (95% CI 301–1144) estimated influenza-attributable deaths in infants aged <2 years, and 1010 (95% CI 742–1630) deaths in children aged ⩾2 years (Table 2). In infants aged <2 years, the estimated influenza-attributable deaths were 2·0 (95% CI 1·1–4·5) times higher than observed during the non-pandemic seasons. In children aged ⩾2 years, estimated influenza-attributable deaths were 2·2 (95% CI 1·5–3·7) times higher during the non-pandemic period. In both age groups, the estimated deaths during the pandemic did not differ significantly from the influenza-certified deaths.
Table 2. Paediatric influenza-certified and estimated influenza-attributable deaths by influenza season and age group, United States, 2003–2004 to 2009–2010
CI, Confidence interval.
* Influenza seasons defined as week 40 to week 39 of the following year, with the exception of the 2008–2009 season (week 40, 2008 to week 16, 2009), and the pandemic period (week 17, 2009 to week 39, 2010).
DISCUSSION
Unlike prior analyses that have focused mostly on adult mortality, our model is based on the observed epidemiology of influenza-associated mortality in children. The influenza-attributable paediatric mortality estimated by this model during the non-pandemic seasons was approximately double the number of influenza-certified deaths. Reports of influenza deaths from death certificates and from laboratory-confirmed case surveillance probably underestimate the true influenza mortality burden; however, these surveillance methods provide valuable information on the timing and relative number of deaths from season to season. Our model uses data on known paediatric influenza deaths and produces influenza mortality estimates that intuitively follow the inter-seasonal trends suggested by available mortality data in the paediatric population. We account for differences in death coding practices during the pandemic and estimate that during the pandemic, in contrast to prior seasons, the majority of the influenza-attributable deaths were recorded as influenza on death certificates.
The number of influenza-attributable deaths relative to influenza-certified deaths differed during the 2009 pandemic period, when relatively few influenza-attributable deaths were estimated to be missed by death certificate reporting compared with the preceding non-pandemic influenza seasons. Death certificate coding practices have been observed to change during the pandemics of 1968–1969 and 1975–1976, when decreases in pneumonia-coded deaths coincided with increases in influenza-coded deaths [Reference Thompson17]. During the 2009 pandemic, many of the influenza-attributable deaths that would have been missed in prior seasons were probably coded as influenza due to a combination of several factors including public awareness and concern [Reference Chew and Eysenbach18], media coverage, diagnostic testing practices [Reference Olowokure19], and increased public health communication with clinicians [Reference Staes20]. Our analysis accounts for differences in coding practice during the pandemic, and future analyses that use a different study period will need to consider the variations in death certificate coding practices from season to season and also over time.
We used a composite group of underlying causes of death that we hypothesized would capture much of the influenza-associated mortality burden in children. We chose not to use all-cause mortality for children because the majority of deaths in persons aged 1–19 years are due to unintentional or intentional injuries [Reference Borse21], which do not have plausible connections to influenza mortality. However, limiting data to underlying respiratory and circulatory deaths as in some previous analyses [Reference Thompson8] may be too restrictive to capture the burden of influenza mortality in children. Among fatalities and hospitalized children, ICD-9 and ICD-10 codes have limited sensitivity for even laboratory-confirmed influenza virus infection [Reference Hoefer3, Reference Keren22]. Prior studies have shown that deaths of various causes occurring in persons with an easily recognizable medical condition will have that condition inappropriately listed as the underlying cause of death [Reference Baird and Sadovnick23, Reference Landes and Peek24]. Neurological comorbidities are common in children with influenza-associated death [Reference Blanton10–Reference Wong12], and it is likely that excluding underlying neurological deaths for this population misses a substantial number of influenza-attributable deaths. Because secondary bacterial infections and sepsis are common complications of influenza infection in children [Reference Wong12, Reference Finelli25], it is also likely that many influenza-attributable deaths are coded with sepsis of bacterial or non-bacterial aetiology as the underlying cause of death. Because influenza-associated deaths can occur very quickly in children, many may present with a brief and fulminant septic or viral syndrome and die before receiving medical care or before diagnostic testing can be performed [Reference Wong12]; in these children, a non-specific underlying cause of death, such as sepsis or an unspecified viral syndrome, may be recorded.
The excess influenza mortality in this analysis is similar to estimates from others that use different parameters and influenza seasons [Reference Foppa and Hossain5, 13]. However, an important characteristic of our model is that the influenza-attributable paediatric mortality follows the number of influenza-certified paediatric deaths from season to season, rather than syndromic or virological influenza indicators gathered from all age groups. A previously published analysis using virological indicators estimates that between 2003 and 2007, most paediatric deaths occurred in the 2004–2005 season [13]; however, in our analysis, the season with most observed influenza-certified mortality (2003–2004) is also the season with the most estimated influenza-attributable mortality. The observed paediatric influenza deaths may also have the advantage of reflecting factors that influence case fatality rates over time, such changes in treatment practices or antiviral availability, which may not be well represented by syndromic or virological surveillance data.
This study has several limitations. A constant relationship between influenza-certified and influenza-attributable mortality is imposed for the six seasons prior to the 2009 pandemic; this is a simplification of the true inter-seasonal variations in death coding practices that probably occur, but it has the advantage of creating a more parsimonious and intuitive model. There are probably influenza-attributable paediatric deaths where the underlying cause is not one that was included in our model; for example, a chronic health condition that did not fall into the defined categories, such as cancer, or a symptom, such as unspecified fever, may have been listed as the underlying cause for some deaths attributable to influenza. Our estimates may be conservative but are likely to be more specific for influenza-attributable death. The number of paediatric influenza-certified deaths has limitations as a surrogate for paediatric influenza mortality because the numbers of deaths are small; other surrogates for mortality that use less rare events, such as medically attended ILI, do not have this limitation. In the age-restricted models, this predictor was too small to be stratified; thus, influenza-certified deaths in children aged 0–17 years are used as a surrogate for influenza mortality trends in children aged <2 and ⩾2 years. This limitation is similar to that of prior models that use indicators of influenza activity in all ages to predict age-specific mortality [Reference Goldstein6, Reference Thompson8, 13]. RSV is an important seasonal cause of severe disease in children [Reference Lloyd26], and previously used methods to separate RSV- and influenza-attributable mortality include examining periods of influenza and RSV predominance separately [Reference Izurieta27], using RSV surveillance data in addition to influenza surveillance data in the model [Reference Tempia7, Reference Thompson8, Reference Pitman28], and stratifying deaths in children and infants [Reference Tempia7]. In this study, we have removed deaths coded as bronchiolitis and have conducted an additional analysis restricting deaths by age <2 and ⩾2 years; however, it is possible that some of the estimated influenza-attributable deaths are attributable to RSV, and that some of the bronchiolitis-coded deaths are attributable to influenza. There has been discussion over the appropriateness of models that define either an exponential or linear relationship between model predictors and mortality data [Reference Goldstein6, Reference Gay29, Reference Thompson30], as well as discussion of different mathematical methods used to fit the seasonal baseline [Reference Foppa and Hossain5, Reference Goldstein6, Reference Thompson30]; our model combines certain methods used in several prior models and ultimately produces a mortality burden estimate that is similar to, and thus helps validate, other approaches.
The true burden of mortality attributable to influenza in children may be almost two times higher than what has been reported during non-pandemic seasons. This highlights the importance of models to derive better estimates of influenza burden in children that can help encourage prevention and treatment strategies against influenza, including influenza vaccination and early and aggressive treatment with influenza antiviral agents in children when indicated [31]. Estimates of paediatric influenza excess mortality should consider the epidemiology of paediatric influenza fatalities and the limitations of generalizing all-age data to paediatric populations when selecting model parameters. Making better use of available influenza surveillance data on children is likely to improve the reliability of excess mortality estimates and allow validation of model outputs.
ACKNOWLEDGEMENTS
We thank Edward Goldstein of the Harvard School of Public Health for his valuable comments on the manuscript. This research received no specific grant from any funding agency, commercial or not-for-profit sectors. The findings and conclusions in this study are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.







