Metabolic syndrome (MetS), characterised by a clustering of metabolic abnormalities, is a multiplex risk factor for morbidity and mortality from type 2 diabetes and CVD, as well as all-cause mortality(Reference Wilson, D’Agostino and Parise1–Reference Eckel, Grundy and Zimmet4). This constellation of metabolic abnormalities, as defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) panel, includes abdominal obesity, high blood pressure (BP), hyperglycaemia, hypertriacylglycerolaemia and low HDL-cholesterol(Reference Eckel, Grundy and Zimmet4,5) . Globally, around one-quarter of the world population (or over one billion people) are living with MetS, representing a major public health threat worldwide(Reference Saklayen6).
Chronic inflammation, a pathogenic feature of atherosclerosis, has been suggested to play a key role in the development and progression of MetS(Reference Haffner7,Reference Koh8) . The level of inflammation can be reduced by lifestyle modifications(Reference Giugliano9). Healthy diets, such as the Mediterranean diets high in fruits, vegetables, nuts and whole grains, have been found to be associated with lower inflammation levels and reduce the risk of MetS(Reference Giugliano9,Reference Ahluwalia, Andreeva and Kesse-Guyot10) . In this context, dietary inflammatory index (DII) was conceptualised as a tool to measure the inflammatory potential of individuals’ diets, with a higher DII representing a more pro-inflammatory diet and a lower DII indicating a more anti-inflammatory diet(Reference Shivappa, Godos and Hébert11,Reference Shivappa, Steck and Hurley12) .
Thereafter, a series of systematic reviews have established the associations between higher DII scores and multiple health outcomes, such as cardiovascular risk and mortality, cancers and depression(Reference Shivappa, Godos and Hébert11,Reference Shivappa, Godos and Hébert13,Reference Wang, Zhou and Chen14) . In 2018, Namazi and colleagues conducted the first systematic review and meta-analysis that examined the association between DII and MetS, where the pooled effect size indicated no statistically significant associations(Reference Namazi15). However, only five studies (two cohorts and three cross-sectional studies) were included in their meta-analysis; the statistical power of analysis could not be well-guaranteed. Nevertheless, the associations between DII and individual MetS components remained unclear, highlighting the need for a more comprehensive systematic review on this topic.
Therefore, we performed a systematic review and meta-analysis of observational studies that assessed the associations of DII with MetS and its components, namely abdominal obesity, high BP, hyperglycaemia, hypertriacylglycerolaemia and low HDL-cholesterol.
Methods
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement(Reference Moher, Liberati and Tetzlaff16).
Search strategy and study selection
From inception to September 2020, a comprehensive literature search was conducted in PubMed, Medline and Embase, using a combination of search terms relating to DII and MetS in titles, abstracts or index term fields. No language or geographic restrictions were applied. The search strategies in the three bibliographic databases are listed in online supplementary material, Supplemental Table S1. The reference list of the last systematic reviews by Namazi and colleagues(Reference Namazi15) was manually searched to ensure that all relevant publications were identified.
For study selection, eligible studies needed to be observational in design (cross-sectional or cohort) and have investigated the associations between DII and MetS in the general population. OR or relative risk (RR) with corresponding 95 % CI or se should have been provided in the included studies. Multiple publications of the same investigation were compared, and the one with the most recent or most comprehensive results was kept. In vitro studies, animal studies, randomised controlled trials and non-original studies (reviews, news and commentaries) were excluded. Studies where participants were purposely selected, such as cancer patients, were excluded.
After the removal of duplicates, two reviewers (Q.Y. and X.L.) independently screened titles and abstracts of retrieved records against the above-mentioned selection criteria. Then, the same two reviewers independently examined the full text of potentially relevant articles. Disagreements in the study selection process were solved through discussion.
Data extraction
From each included article, two independent reviewers (Q.Y. and X.L.) extracted the following information: author(s), year of publication, study location and country, WHO region (as African Region, Region of the Americas, South-East Asia Region, European Region, Eastern Mediterranean Region and Western Pacific Region), World Bank income region (as high-income countries and low- and middle-income countries), investigation year for cross-sectional studies or study period for cohort studies, the definitions of DII and MetS, the effect estimates (OR in cross-sectional analyses or RR in cohort analyses) and its uncertainty, the comparison level, and covariates adjusted for in multivariable analyses. For studies that reported crude and multivariable-adjusted OR or RR, we extracted the effect estimates from multivariable models that adjusted for the most potential confounding factors.
Quality assessment
Two reviewers (Q.Y. and Y.H.) independently assessed the quality of included articles using the Quality Assessment Tool for Observational Cohort and Cross-sectional Studies. The cross-sectional studies were evaluated on ten points, and cohort studies were on fourteen points. According to the overall quality scores, included articles were categorised into low- (cross-sectional: ≤3 points; cohort: ≤5 points), medium- (cross-sectional: 4–7 points; cohort: 6–9 points) and high-quality groups (cross-sectional: 8–10 points; cohort: 10–14 points)(17).
Data synthesis and analysis
The associations between DII and MetS were evaluated with different effect estimates and comparison groups across included articles, such as OR or RR for per-unit or per-sd change, or for comparisons of the extremes of halves, tertiles, quartiles or quintiles. To ensure a consistent reporting approach, we referred all effect estimates as OR. According to the approach proposed by Chêne and colleagues, different comparison groups were transformed and harmonised to enable meta-analysis(Reference Chêne and Thompson18). All reported OR and RR were converted into OR of the top against bottom quartiles. First, OR were logarithmically transformed to normalise the distribution; then, ln(OR) was rescaled with the comparison between the top v. bottom quartiles being equivalent to 2·54 times the change for 1 sd, 2·54/2·18 times the comparison between the top v. bottom tertiles and 2·54/2·80 times the comparison between the top v. bottom quintiles(Reference Chêne and Thompson18). In articles where effect estimates were presented separately for males and females, those were included in the overall meta-analysis as distinct data points.
An inverse-variance weighted random effects (DerSimonian and Laird method) meta-analysis was adopted to pool the summary OR. In addition, sensitivity analyses were conducted by only including individual studies with high quality. Publication bias was evaluated by visual inspection of the funnel plot and the Egger’s regression test for asymmetry (when ten and more study points were available)(Reference Egger, Smith and Schneider19,Reference Peters, Sutton and Jones20) .
Heterogeneity between studies was assessed and quantified with Cochran’s Q test and the I 2 statistic. In Cochran’s Q test, a P-value of <0·05 indicated statistically significant heterogeneity. In I 2 statistic, a value larger than 50 % represented a high degree of heterogeneity(Reference Barendregt, Doi and Lee21,Reference Higgins and Green22) . To explore potential sources of heterogeneity, subgroup meta-analysis and meta-regression were performed. A priori defined group variables included study design (cross-sectional v. cohort), WHO region, World Bank region, DII definition, diagnostic criteria for MetS and adjustment of covariates (including BMI, physical activity and total energy intake).
The analyses were conducted for the associations of DII with MetS and its five components, namely abdominal obesity, high BP, hyperglycaemia, hypertriacylglycerolaemia and low HDL-cholesterol, separately. The level of statistical significance was set as P < 0·05. Data were analysed using STATA version 14.0.
Results
Study selection and characteristics
Of 279 records identified by the initial searches, thirty were assessed in full text. Finally, eighteen articles, published between 2014 and 2020, explored the associations between DII and MetS were deemed eligible. Among the eighteen articles, thirteen articles additionally provided information on the associations between DII and the five MetS components, one on the associations of DII with abdominal obesity, hyperglycaemia, hypertriacylglycerolaemia and low HDL-cholesterol, one on the associations of DII with abdominal obesity and high BP. Three articles did not explore the association between DII and individual MetS components (Fig. 1). The characteristics of the included articles are provided in Supplemental Table S2. The majority of the included articles were based on cross-sectional analyses (n 14, 77·8 %), adopted the DII definition by Shivappa (n 16, 88·9 %) or defined MetS using the NCEP-ATP III criteria (n 11, 61·1 %). The regions where the included articles were investigated included European Region (n 6, 33·3 %), Eastern Mediterranean Region (n 5, 27·8 %), Region of the Americas (n 4, 22·2 %), South-East Asia Region (n 2, 11·1 %) and Western Pacific Region (n 1, 5·6 %). Half of the included articles were conducted in high-income countries, and half were in low- and middle-income countries. The quality assessment of each included article is listed in Supplemental Table S3, and the majority of included articles were with high quality (n 16, 88·9 %).
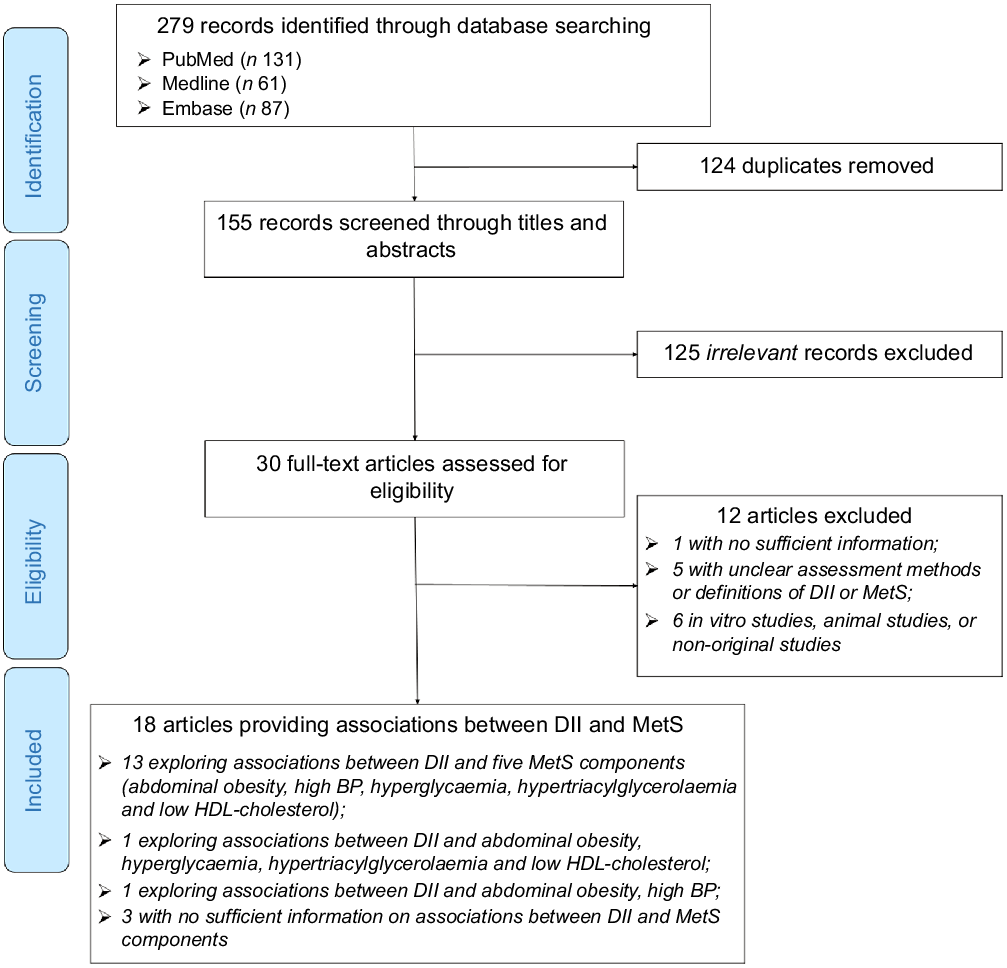
Fig. 1 Flow diagram of study selection process
Dietary inflammatory index and metabolic syndrome and its components
As two articles provided effect estimates for males and females separately, a total of twenty data points were available for data synthesis from the included eighteen articles. As shown in Table 1 and Fig. 2, the summary OR for the association between top v. bottom quartile of DII and MetS was 1·23 (95 % CI 1·10, 1·38) based on 20 data points. A significant high degree of heterogeneity among the included data points was revealed (I 2 = 70·2 %, p < 0·001). There was no significant publication bias (Egger’s test, P = 0·084, see online supplementary material, Supplemental Figure S1).
Table 1 Summary effects and 95 % CI using random effects meta-analysis for the associations of dietary inflammatory index (DII) (top v. bottom quartiles) with metabolic syndrome (MetS), stratified by study characteristic
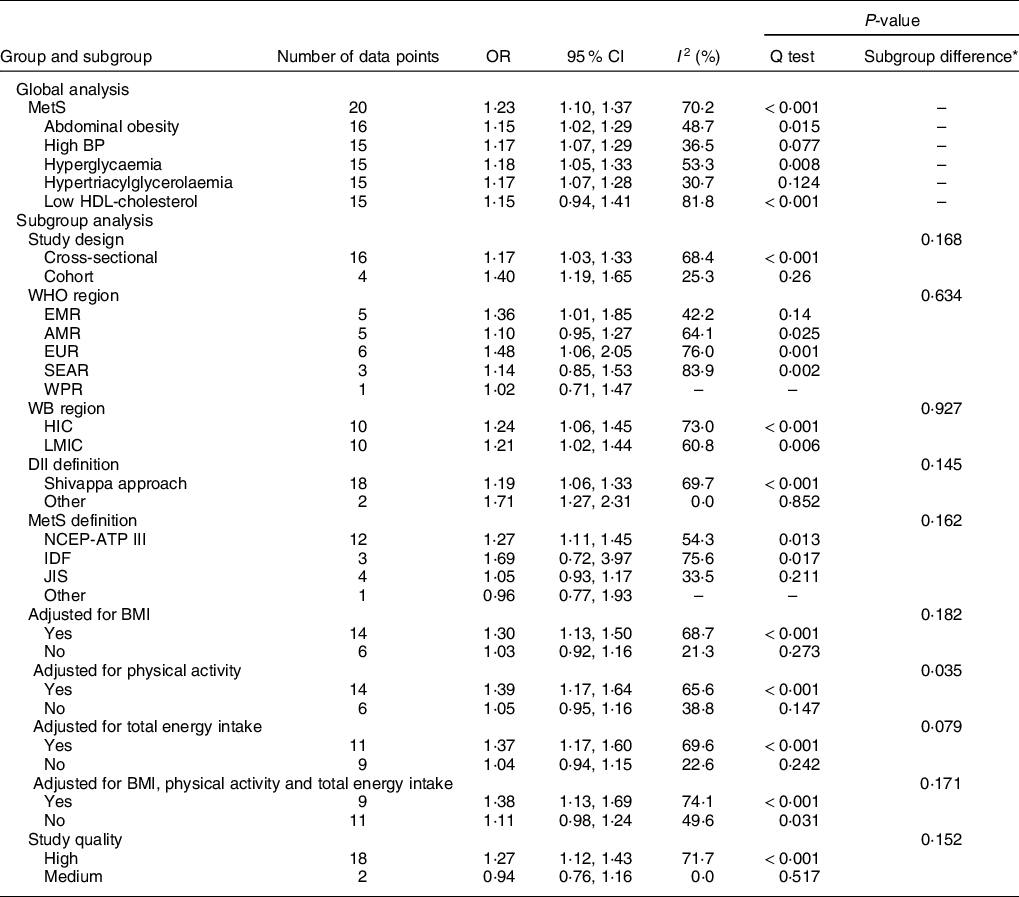
BP, blood pressure; EMR, Eastern Mediterranean Region; AMR, Americas Region; EUR, European Region; SEAR, South-East Asia Region; WPR, Western Pacific Region; HIC, high-income countries; LMIC, low- and middle-income countries; NCEP-ATPIII, The Third Report of the National Cholesterol Education Program Expert Panel (NCEP) on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III); IDF, The International Diabetes Federation Criteria; JIS, Joint Interim Statement.
* P values for heterogeneity between subgroups were based on meta-regression analyses; two articles provided effect estimates for males and females separately; therefore, the total number of data points from the eighteen included articles were 20.
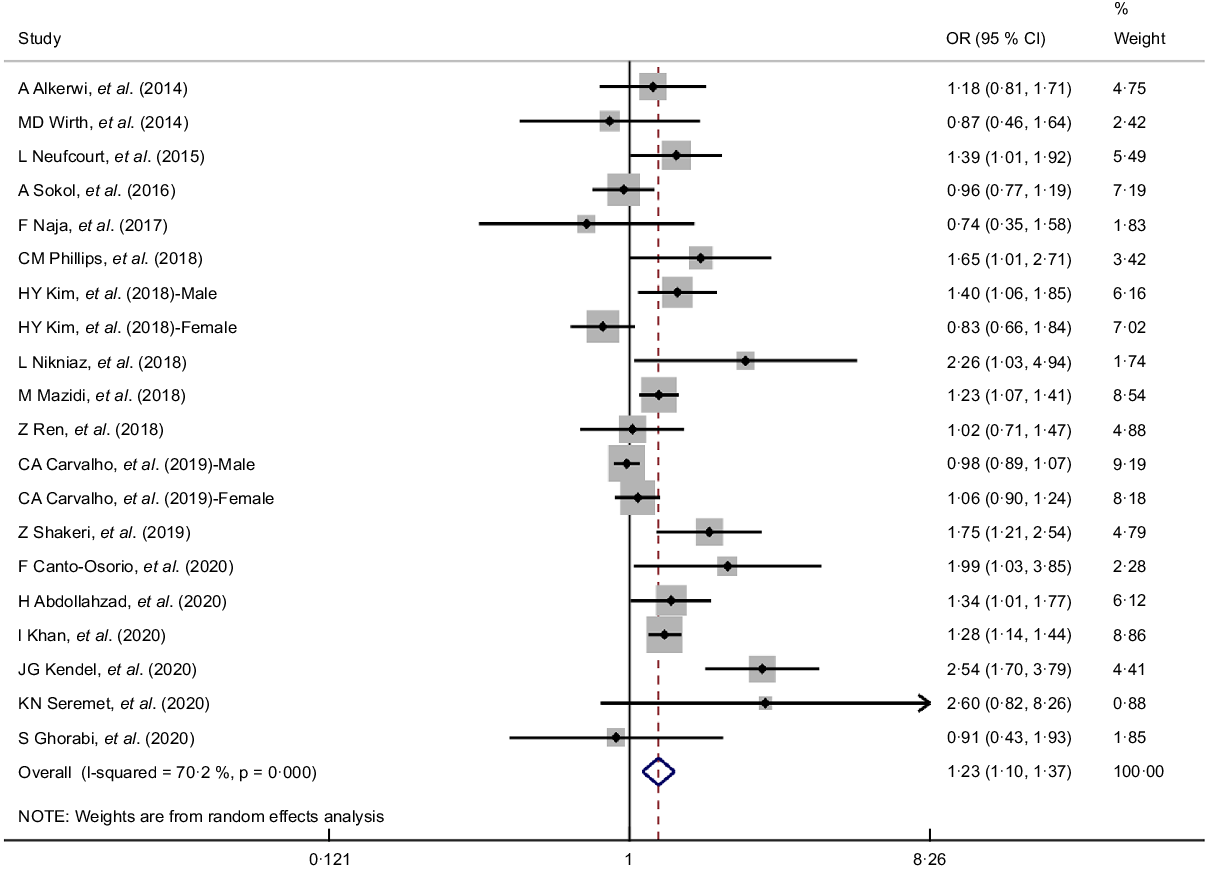
Fig. 2 Forest plots (random effects model) of meta-analysis on the association between dietary inflammatory index (DII) and metabolic syndrome (MetS) (top v. bottom quartiles)
Moreover, people in the top quartile of DII had significant higher odds of MetS components, except for low HDL-cholesterol, whose summary OR was 1·15 (95 % CI 0·94, 1·41) based on fifteen data points. The summary OR comparing top v. bottom quartiles of DII were 1·15 (95 % CI 1·02, 1·29) for abdominal obesity, 1·17 (95 % CI 1·07, 1·29) for high BP, 1·18 (95 % CI 1·05, 1·33) for hyperglycaemia and 1·17 (95 % CI 1·07, 1·28) for hypertriacylglycerolaemia. Significant heterogeneity was revealed in the meta-analyses of associations between DII (top v. bottom quartiles) and abdominal obesity (I 2 = 48·7 %, P = 0·015), hyperglycaemia (I 2 = 53·3 %, P = 0·008) and low HDL-cholesterol (I 2 = 81·8 %, P < 0·001). According to funnel plots and Egger’s tests, no publication bias was detected in the meta-analyses for all MetS components (see online supplementary material, Supplemental Figure S2–S6).
Sub-group, meta-regression and sensitivity analyses
The results for sub-group meta-analyses and meta-regressions of the association between DII (top v. bottom quartiles) and MetS are listed in Table 1. With regard to study design, cohort studies yielded a slightly higher summary OR than cross-sectional studies (1·40 (95 % CI 1·19, 1·65) v. 1·17 (95 % CI 1·03, 1·33)); however, the difference was not statistically significant (P = 0·168 according to meta-regression). When only based on cohort studies, the summary OR demonstrated positive associations between higher DII category and all MetS components (see online supplementary material, Supplemental Table S4 for more details).
We assessed the association between DII (top v. bottom quartiles) and MetS in different regions and found that the associations tended to be similar between low- and middle-income countries and high-income countries, but differ among WHO regions, with only Eastern Mediterranean Region and European Region yielding significant associations. Regardless how DII was defined, the association between DII (top v. bottom quartiles) and MetS remained positive. However, when MetS was defined according to criteria other than NCEP-ATP III, no statistically significant associations were revealed. When adjustments were made for BMI, physical activity, total energy intake or a combination of BMI, physical activity and total energy intake, the associations between DII (top v. bottom quartiles) and MetS were statistically significant. However, when no such adjustments were made, no statistically significant associations were revealed.
Based on meta-regressions, the summary OR did not change significantly by study characteristic, including study region (WHO region or World Bank region), DII and MetS definitions, and whether adjustments were made for BMI, total energy intake or a combination of BMI, physical activity and total energy intake (P ≥ 0·05 for all comparisons). Significant difference in summary OR was noted between studies that adjusted for physical activity and those that did not (P = 0·035), with studies that had accounted for the effect of physical activity yielding a significant association between DII (top v. bottom quartiles) and MetS (summary OR: 1·39 (95 % CI 1·17, 1·64)). In the sensitivity analysis by only including studies with high quality, the summary OR was slightly changed to 1·27 (95 % CI 1·12, 1·43).
Discussion
This systematic review and meta-analysis gathered all available observational studies on the associations of DII with MetS and its components and provides the most comprehensive evidence in this research field. Based on eighteen articles, our results overturn the previous conclusion that there was no significant association between DII and MetS and supports strong positive association between higher DII and MetS(Reference Namazi15). Individuals with more pro-inflammatory diets, as estimated by the highest quartile (v. the lowest quartile) of DII, had 23 % increased risk of MetS (OR: 1·23 (95 % CI 1·10, 1·37)), 15 % increased risk of abdominal obesity (OR: 1·15 (95 % CI 1·02, 1·29)), 17 % increased risk of high BP (OR: 1·17 (95 % CI 1·07, 1·29)), 18 % increased risk of hyperglycaemia (OR: 1·18 (95 % CI 1·05, 1·33)) and 17 % increased risk of hypertriacylglycerolaemia (OR: 1·17 (95 % CI 1·07, 1·28)). However, the positive association between higher DII and low HDL-cholesterol was not statistically significant based on all the included observational studies (twelve cross-sectional and three cohort data points), but turned to be significant when only based on cohort studies. The association between the inflammatory potential in diets and low HDL-cholesterol needs further confirmation, especially in longitudinal studies.
DII was initially designed to capture dietary indices and patterns from the aspect of inflammation. The most widely adopted criteria for defining DII was proposed by Shivappa and colleagues, which contains a total of forty-five dietary components including common food items, macro- and micronutrients and important bioactive polyphenols based on 1943 peer-reviewed publications(Reference Shivappa, Steck and Hurley12). Recent investigations have revealed a significant relation between pro-inflammatory diet (higher DII) and common inflammatory markers, such as C-reactive protein and IL-6(Reference Shivappa, Hébert and Rietzschel23–Reference Shivappa, Wirth and Hurley25). The abnormal increase of C-reactive protein could stimulate monocytes to secrete more pro-inflammatory cytokines, which subsequently lead to a serine phosphorylation of insulin receptor substrate proteins, and therefore, increase insulin resistance from insulin-sensitive tissue(Reference Lopez-Garcia, Schulze and Fung26,Reference Schulze, Hoffmann and Manson27) . Additionally, high levels of IL-6 might decrease the expression and activation of nitric oxide synthase, increase the synthesis of endothelin-133 or impair insulin signalling and activity(Reference Jayedi, Rahimi and Bautista28–Reference Yudkin, Kumari and Humphries30). Given the above-mentioned links, it is not surprising to observe such a positive association between pro-inflammatory diet (higher DII) and MetS in our study. Moreover, the effects of this positive association were similar in both high-income countries and low- and middle-income countries settings, but varied substantially among different WHO regions, which might be a reflection of different diet patterns in different geographic regions and ethnicities. Evidence on the associations of pro-inflammatory diet with the development of MetS and components in different regions and populations is still needed.
Regardless how DII was formulated, the positive association between higher DII and MetS persisted in our meta-analysis. However, such positive association was only observed when MetS was defined according to the NCEP-ATP III criteria. Such results promoted the adoption of a unified and widely acknowledged MetS definition in future meta-analysis in this topic. In nutrition epidemiological studies, it is rather common to make adjustments for confounding factors, such as energy intake, physical activity, medication intake, etc. In our meta-analyses, associations between higher DII and MetS were only positive when adjustments were made for BMI, physical activity, total energy intake or a combination of BMI, physical activity and total energy intake, but became non-significant when adjustments for those covariates were not made. This phenomenon underlines the importance of adjustment for confounding factors in future epidemiological studies in this research field.
To the best of our knowledge, this study provides the most up-to-date and comprehensive assessments on the associations of DII with MetS and components. This study benefits from a comprehensive literature search in multiple bibliographic databases. A total of eighteen unique articles, consisting of twenty data points, were included in our meta-analyses, which represents a substantial improvement in this research field than the previous meta-analysis of only five studies by Namazi and colleagues(Reference Namazi15). Moreover, the relatively larger volume of information made it possible for us, for the first time, to separately explore the associations between DII and individual MetS components.
However, several limitations should be considered when interpreting the findings of this study. First, meta-analysis of observational studies is prone to bias that are inherent in the original studies designs. The majority of included studies in this systematic review and meta-analysis were cross-sectional in design, where temporal associations were not available. When only based on a limited number of cohort studies (four for MetS and three for all MetS components), the associations of higher DII with MetS and its components were all significantly positive and stronger than the effects based on cross-sectional studies. The differences in summary effects driven from study design largely support our hypothesis that chronic inflammation induces the development of MetS and components; however, more prospective studies are still needed to further confirm this conclusion. Second, the importance of accounting for potential confounding factors when assessing the association between DII and MetS was demonstrated in our subgroup meta-analyses; however, due to the lack of relevant information, the effects of only a limited number of covariates (BMI, physical activity and total energy intake) were explored. Third, the substantial heterogeneity across studies was not fully eliminated with subgroup meta-analyses by common study characteristic. Improved analyses should be conducted when more information becomes available in the foreseeable future.
Conclusion
To conclude, results from this systematic review and meta-analysis demonstrate that higher DII is positively associated with MetS and its components including abdominal obesity, high BP, hyperglycaemia and hypertriacylglycerolaemia. The association between DII and low HDL-cholesterol needs further exploration when more relevant information becomes available. This study indicates the risk of pro-inflammatory diet in the progression of MetS, serving as the basis of dietary interventions for preventing MetS.
Acknowledgements
Acknowledgements: None. Financial support: This research received no external financial support. Conflict of interest: None. Authorship: P.S. planned the study; P.S. and Y.H. designed the methods. Q.Y. and X.L. contributed to the literature review. Q.Y. and Y.H. extracted data. P.S., W.X., J.S. and Z.Y. conducted statistical analyses. P.S. prepared the first draft with important contributions from all other authors. All authors interpreted results, commented on drafts of the paper and approved the final version. Ethics of human subject participation: None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980021000288






