Organisms of the genus Achromobacter are nonfermentative, gram-negative rods that have been linked to outbreaks in the healthcare setting. Reference Mandell, Dollin and Mandell1 They are ubiquitous in water and soil environments. Reference Mandell, Dollin and Mandell1 Achromobacter spp are able to persist in the environment due to their ability to form biofilms. Reference Günther, Merle, Frank, Gaida and Mutters2 They are infrequently isolated from healthcare-associated infections and are often misidentified. Reference Mandell, Dollin and Mandell1,Reference Fisher3 Achromobacter spp are opportunistic pathogens, causing infections in immunocompromised hosts. Reference Fisher3 However, a number of case reports have described Achromobacter infections in immunocompetent hosts. 4
Clinicians remain poorly informed regarding these bacteria and they are often regarded as contaminants. 4 This genus includes several species, and A. xylosoxidans is the most common clinical organism, associated with outbreaks of nosocomial infections. Reference Fisher3 Outbreaks have been linked to contaminated solutions, such as intravenous fluid, soaps, disinfectants, incubators, and humidifiers. Reference Mandell, Dollin and Mandell1 Neonatal infections resulting from perinatal transmission of Achromobacter from the mother to the neonate have been reported. Reference Mandell, Dollin and Mandell1
Achromobacter spp are intrinsically resistant to numerous antibiotics. Reference Isler, Kidd, Stewart, Harris and Paterson5 Resistance is mediated by multidrug efflux pumps, constitutive oxacillinases, as well as acquired β-lactamases. Reference Mandell, Dollin and Mandell1,Reference Isler, Kidd, Stewart, Harris and Paterson5 Genus-specific susceptibility testing methods for Achromobacter spp are lacking. Reference Isler, Kidd, Stewart, Harris and Paterson5 The Clinical and Laboratory Standards Institute (CLSI) has provided minimum inhibitory concentration (MIC) break points for the Achromobacter spp under the ‘other non-Enterobacterales’ category. 6 Treatment of Achromobacter spp is guided by the susceptibility profile of the isolated organism, with seemingly good correlation between in vitro and in vivo results. Reference Mandell, Dollin and Mandell1,Reference Isler, Kidd, Stewart, Harris and Paterson5
In June 2018, at the Tshwane Academic Microbiology (TAD) laboratory (Pretoria, South Africa), registrars noticed an increase in the number of A. denitrificans isolates identified from clinical specimens. During the second 6 months of 2017, the laboratory identified zero A. denitrificans isolates whereas the laboratory identified 10 isolates in the first 6 months of 2018. Here, we describe the investigation of an outbreak of A. denitrificans at a tertiary-care hospital in Pretoria, South Africa.
Methods
Following confirmation of the outbreak, the laboratory proceeded to create a line list of the affected patients. The laboratory also included a questionnaire distributed to the registrars who came across any isolate of A. denitrificans on their respective benches in the microbiology laboratory. The information requested on these questionnaires included date of isolation of the A. denitrificans; site of isolation of the organism (e.g., blood culture); demographics of the patient such as age, sex and location of the patient; details of whether the patient had clinical signs of sepsis (i.e., fever, tachycardia, low blood pressure, raised procalcitonin, C-reactive protein, and white cell count); treatment given; and response of the patient to treatment. The questionnaire also probed whether the patient had any malignancy or other immunocompromising condition, whether any catheters were present, whether the patient was receiving dialysis (peritoneal or hemodialysis), and any other significant background history. The blood culture bottle lot number was also requested for each case of bloodstream infection to investigate whether the bottles were the source of the contamination.
The A. denitrificans isolates that were cultured from clinical specimens were identified using the Vitek 2 (bioMèrieux, Marcy-l’Étoile, France) automated system. Antimicrobial susceptibility testing (AST) was performed using the Kirby-Bauer disk diffusion method. Results were interpreted according to the break points for Pseudomonas aeruginosa, according to the Clinical Laboratory and Standards Institute (CLSI) 2018 M-100 document. 6 We chose to use Pseudomonas aeruginosa break points because break points for “other non-Enterobacterales” only provide minimum inhibitory concentrations (MICs), which we could not obtain for most of the drugs.
If the isolate was cultured from blood (n = 27), the isolate was reported to the clinicians as a gram-negative bacillus following the Gram stain. A final report was issued once the full identification and AST results were available. The attending clinicians were also called for each isolate to determine the diagnosis of these patients as well as the clinical relevance of the cultured isolates.
With each new case identified, the attending clinicians were interviewed to determine a common source for the isolates cultured. A common finding of these interviews was that all clinicians used a chlorhexidine-and-water solution (prepared in the pharmacy on site, batch numbers 180615/01, 181128/03, 1811203/04, 181211/02) for skin antisepsis, urethral catheterization, as well as for wound care. This solution was prepared in house by the hospital pharmacy staff.
In the hospital pharmacy, the solution preparation area, various other items, and the solution were all sampled using moistened swabs. These sites included the table-top surfaces in the pharmacy where the chlorhexidine-and-water solution was prepared, surfaces of the bottles that were used to package the chlorhexidine-and-water solutions, the exterior and interior surfaces (including nozzles) of the dispenser device (Fig. 1), the bromothymol blue (prepared in the pharmacy on site, batch no. 29945), the 0.05% chlorhexidine stock solution (B Braun Medica, Randburg, South Africa), and sterile water (B Braun Medical, Randburg, South Africa, batch no. 18Y542B). Multiple samples of the chlorhexidine-and-water solutions were also collected for microbiological culture. These specimens were all plated qualitatively on 5% sheep blood agar, chocolate agar, and MacConkey agar and were incubated at 35–37°C in ambient air. The results were read after 48 hours of incubation.

Fig. 1. The dispenser device used by the pharmacy to prepare the chlorhexidine and water solution. The chlorhexidine, water and bromothymol blue mixture are placed in the bucket on the floor, then siphoned up by the hose and filled into empty bottles which are placed beneath the nozzle.
The first 16 A. denitrificans isolates cultured from different patients were subjected to repetitive-element, sequence-based, polymerase chain reaction (rep-PCR) to determine the clonality of these isolates. Total genomic DNA from A. denitrificans was used as a template in the rep-PCR assay. The primer-pair sequences REP1 (5’-IIIGCGCCGICATCAGGC-3’) and REP 2 (5’-ACGTCTTATCAGGCCTAC-3’) used in this study have been described previously. Reference Iraz, Özad Düzgün and Sandallı7 The rep-PCR reaction was performed as follows: 1 µL of template DNA was added to 12.5 µL MyTaq HS mix (Bioline, London, UK) with 0.4 µM of each primer and made up to 25 µL using nuclease-free water. The rep-PCR was performed in a thermocycler under the following conditions: an initial denaturation of 94°C for 3 minutes, followed by 30 cycles of 94°C for 45 seconds, 45.8°C for 1 minute, and 72°C for 8 minutes, with a final extension step of 72°C for 16 minutes. The rep-PCR products were visualized and separated using gel electrophoresis for 3 hour 20 minutes at 80 volts. A 1-kb Plus Ready-to-Use DNA Ladder (Thermo Fisher Scientific, Waltham, MA) was used to compare the banding patterns in each strain. Isolates were assigned to the same rep-PCR group if their pattern differed by <3 bands, according to previously defined criteria. Reference Spigaglia and Mastrantonio8
Results
Achromobacter denitrificans was isolated from clinical specimens obtained from 43 patients during the outbreak period between February and December 2018. Figure 2 depicts the sites from which the organisms were cultured. Table 1 provides details of the risk factors associated with A. dentrificans infection as well as antibiotic treatment in affected patients, as obtained from the questionnaires.
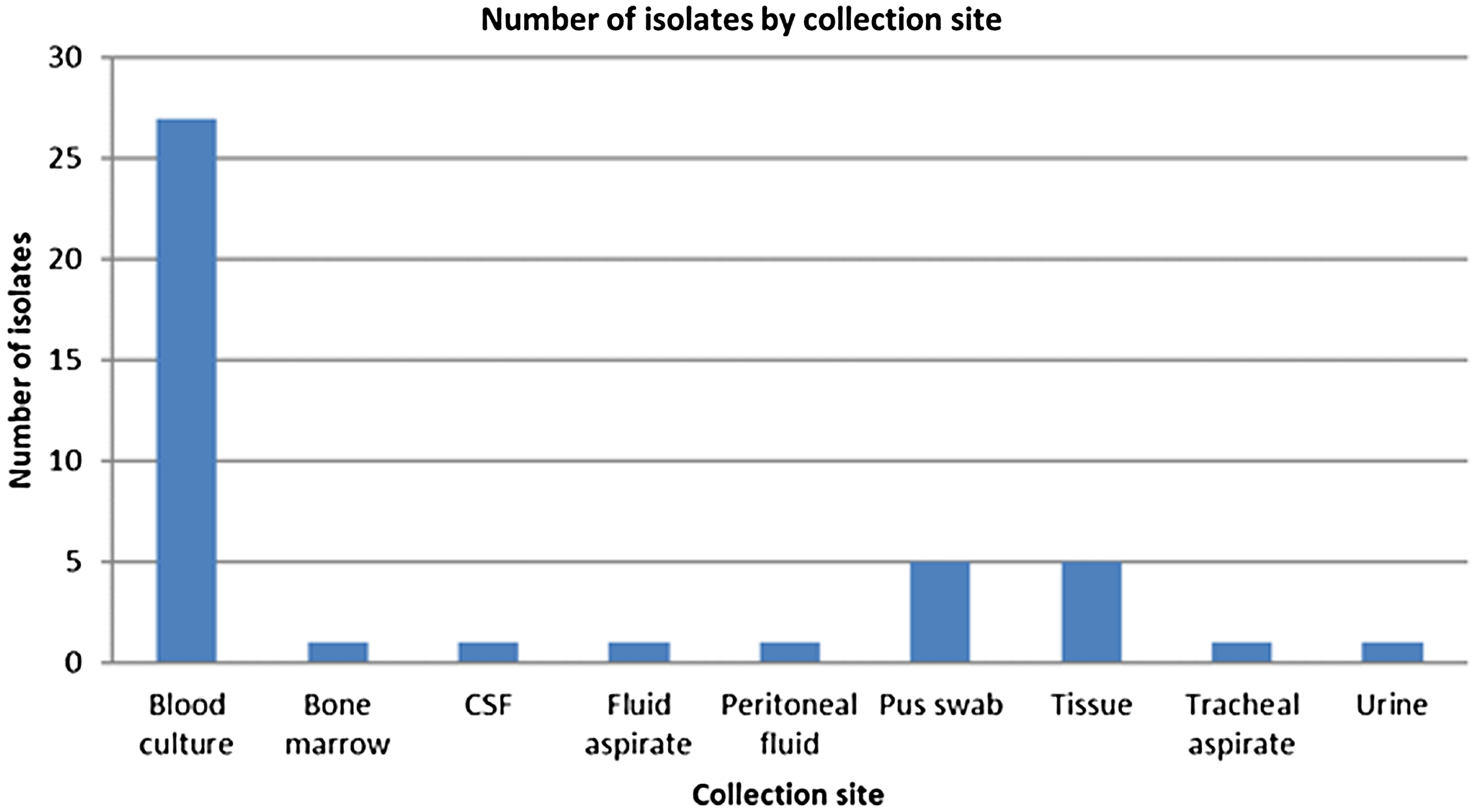
Fig. 2. Anatomical sites where A. denitrificans was isolated.
Table 1. Risk Factors and Antibiotic Usage Associated With Infection Caused by Achromobacter dentrificans

*Mean value and range reported.
The AST pattern of these isolates was identical in all the strains tested. The isolates were resistant to aztreonam and the aminoglycosides but were susceptible to the antipseudomonal agents, namely piperacillin-tazobactam, ceftazidime, cefepime, imipenem, meropenem, as well as the fluoroquinolones.
The clinical diagnosis of the affected patients was available for 22 patients, but we could not determine a diagnosis in 21 patients, mainly because we could not reach the attending clinician. The diagnoses in these patients as well as the patient’s inflammatory markers are listed in Table 2.
Table 2. Diagnoses of Patients Infected with A. denitrificans During the Outbreak and Their Inflammatory Markers
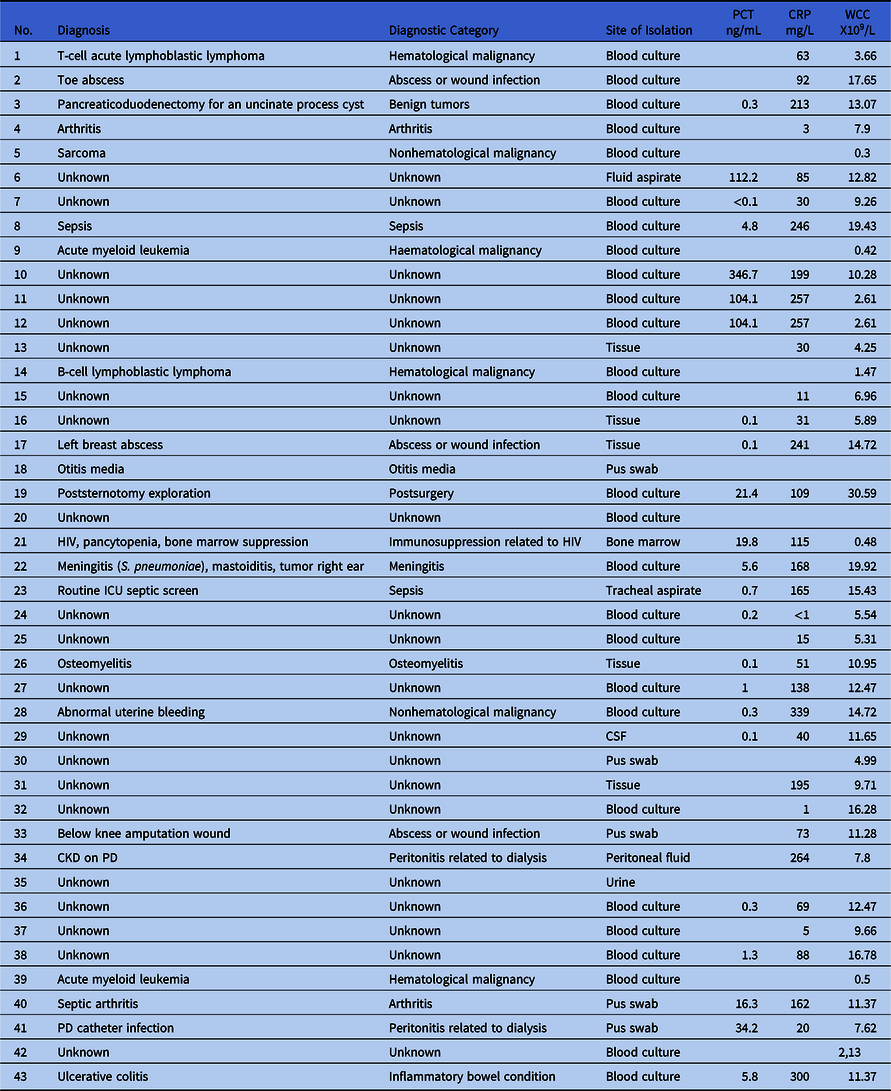
Note. PCT, procalcitonin; CRP, C-reactive protein; WCC, white cell count; HIV, human immunodeficiency disorder; ICU, intensive care unit; CSF, cerebrospinal fluid; CKD, chronic kidney disease; PD, peritoneal dialysis.
The isolates cultured were distributed widely throughout the Steve Biko Academic Hospital (SBAH). Interestingly, the isolates were restricted to the SBAH, despite the TAD microbiology laboratory servicing 2 other tertiary-care hospitals, 2 secondary-care hospitals, and several primary-care clinics. The problem seemed to occur only at SBAH. Figure 3 depicts the wards where these isolates were cultured at SBAH.

Fig. 3. Location of A. denitrificans cultured from the SBAH.
A potential source for the outbreak was thought to be the chlorhexidine-and-water solution prepared by the hospital pharmacy. The hospital pharmacy and the site where the solution was prepared were investigated. Sterile water was mixed with equal amounts of chlorhexidine 0.05% solution. A small volume of bromothymol blue was added to the solution to give a distinctive color to make it identifiable to the hospital staff. The solution was made in large batches then aliquoted using a dispenser device (Fig. 1), then it was packaged into sterile plastic bottles.
All cultures of the chlorhexidine-and-water solution as well as swabs taken form the interior surfaces and nozzles of the dispenser device grew A. denitrificans. No bacterial growth was detected on any of the other specimens collected from the hospital pharmacy.
In addition, 16 A. denitrificans strains obtained from clinical isolates were subjected to rep-PCR genotyping to determine the genotypic relatedness between the strains (Fig. 4). The strains were visually classified in 2 genotypes. Most of the strains conformed with 1 genotype; only 4 strains conformed with the other genotype.
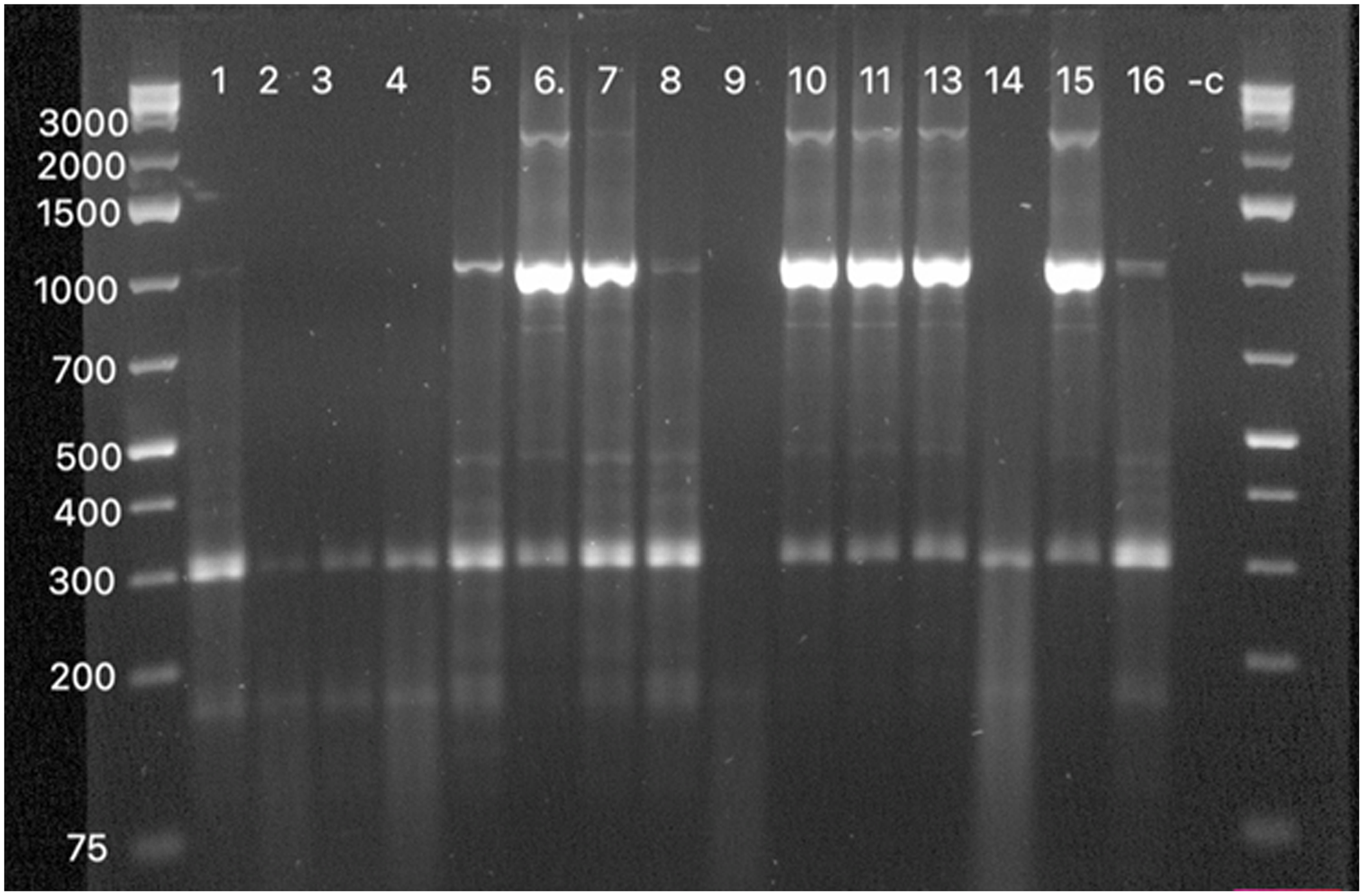
Fig. 4. DNA banding patterns obtained for the A.denitrificans strains by rep-PCR. The first and last lanes shows the 1- kb Plus DNA Ladder. The numbers on the left are the sizes of the DNA ladder in base pairs. The numbers labeled 1–16 are the strains that were studied. The −c refers to the negative control used in this assay.
Discussion
Compared with the baseline number of cases that the SBAH laboratory usually sees, the fact that our laboratory cultured 43 isolates of A. denitrificans from clinical specimens over an 11-month period clearly indicated an outbreak. A chlorhexidine-and-water solution is used at SBAH for skin antisepsis, to clean wounds and abrasions, and as a disinfectant prior to urinary catherization. The Achromobacter isolates were cultured from a wide range of sources at the SBAH. The fact that this organism can survive in a wide range of disinfectants allows the organism to survive and colonize or infect surfaces and explains the range of sites where the organism was cultured. This environmental organism has been shown to colonize reusable plastic containers and to display resistance to common disinfectants, such as chlorhexidine, and particularly to quaternary ammonium compounds. Reference Molina-Cabrillana, Santana-Reyes, Gonzalez-Garcia, Bordes-Benitez and Horcajada9,Reference Tena, Carranza, Barbera, Valdezate, Garrancho and Arranz10 The organism may even contaminate ward consumables such as ultrasound gel, and outbreaks have been reported in such instances. Reference Haviari, Cassier and Dananche’11
Achromobacter spp cause infections in patients with underlying diseases and is considered an opportunistic pathogen. Reference Turel, Kavuncuoglu, Hosaf, Ozbek, Aldemir, Uygur, Hatipoglu and Siraneci12 The literature has reported Achromobacter spp to be a cause of bloodstream infections, meningitis, pneumonia, osteomyelitis, soft-tissue infections, infective endocarditis, peritonitis, urinary tract infections as well as ocular infections. Reference Aisenberg, Rolston and Safdar13,Reference Ahmed, Nistal, Jayan, Kuduvalli and Anijeet14 To our knowledge, only 1 previous study published in Columbia has described an outbreak with A. denitificans caused by contaminated chlorhexidine. Reference Echeverri, Atehortúa, Tamayo, Restrepo and Valencia15
In this study, most patients with a known diagnosis had serious underlying conditions. These included arthritis, benign tumors, malignancy, human immunodeficiency virus (HIV), inflammatory bowel disease, osteomyelitis, peritonitis and patients who had undergone a surgical procedure.
The clinicians started these patients on treatment and, in most cases, in the form of a carbapenem such as imipenem or meropenem. Most of the isolates were cultured from invasive sites (n = 37), which is problematic because clinicians are bound to start carbapenem treatment at the SBAH when a gram-negative bacillus is reported on the Gram stain of the specimen. This practice may result in driving antimicrobial resistance and particularly increasing the rate of carbapenemase-producing Enterobacterales (CPE).
Although the inflammatory markers were not elevated in some of these patients (Table 2), in most cases the patients were started on empiric treatment due to the invasive nature of the specimens. Once the antibiograms were available to the clinicians, patients were, in some cases, de-escalated to piperacillin-tazobactam. In other cases, the carbapenem was continued or antibiotics were stopped because the isolates were deemed to be colonizers by the clinicians. In general, Achromobacter spp are resistant to most β-lactams (excluding antipseudomonal agents and carbapenems) and aminoglycosides, whereas the quinolones have poor activity against this organism. Reference Turel, Kavuncuoglu, Hosaf, Ozbek, Aldemir, Uygur, Hatipoglu and Siraneci12,Reference Almuzarra, Limansky, Ballerini, Galanternik, Famiglietti and Vay16 Useful agents in the treatment of Achromobacter spp include trimethroprim-sulfamethoxazole, imipenem, meropenem, as well as piperacillin tazobactam. Reference Ramos, Domine, Ponte and Soriano17
The genotypic analysis by rep-PCR results proved that most patients had the same strain that was circulating in the hospital. In this study, we used rep-PCR because it is simple, rapid, and less expensive to study genotypic relatedness among the strains. Singh et al Reference Singh, Biswal and Hallur18 showed how rapid, useful, and inexpensive this method is and how it can be used in poor-resource settings to control healthcare outbreaks.
The source of the outbreak in our case was the chlorhexidine-and-water solution prepared in the hospital pharmacy. In early January 2019, the microbiology laboratory had communicated with the hospital management and pharmacy to recall and destroy all chlorhexidine-and-water solutions from the wards and outpatient clinics. It was further advised that the dispenser used for the preparation of the solution be replaced or sterilized appropriately by the suppliers.
The pharmacy and hospital management heeded the call from the laboratory and discarded all stock of the chlorhexidine-and-water solution. Chlorhexidine 0.05% solution (without any dilution) was replaced as the agent for skin antisepsis and wound cleaning. There was concern from the wards and surgery theatres regarding the use of chlorhexidine directly for urinary catheter insertion. The microbiology laboratory subsequently advised that sterile saline can be used for meatal cleaning. Whether an antiseptic or sterile saline is superior remains to be established. Reference Gould, Umscheid, Agarwai, Kuntz and Pesueo19 Following removal of the product from the hospital, the microbiology laboratory did not culture any new isolates of A. denitrificans in the first 6 months of 2019. This was taken as proof that the source of the outbreak had been successfully eliminated.
The strengths of the study were the early detection of the outbreak as well as the prompt and thorough investigation to search for the source of the outbreak. Furthermore, once the source was confirmed, the laboratory took firm action to remove the source and end the outbreak. In many such outbreaks, the source of the outbreak is often never found.
This study also had several limitations. We did not do more extensive molecular work on the isolates. We chose rep-PCR on the initial 16 clinical isolates collected over other more discriminatory typing methods because of the ease of availability and due to financial constraints in a resource-limited setting. A further limitation of this study is the lack of follow-up on the outcomes of infected patients.
In conclusion, surveillance in the microbiology laboratory is crucial for rapid identification of outbreak sources. This procedure will prevent patients from unnecessarily being exposed to intravenous antibiotics and will contribute to the prevention of increases in antimicrobial resistance in the hospital.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.









