Introduction
Adult intestinal tract contains two primary smooth muscle layers (SML), namely the muscularis mucosae and the muscularis propria.Reference Watson, Mahe and Munera1–Reference Kobayashi, Khalil and Lei4 Muscularis mucosae is a smooth muscle layer of two to three cells thick located between the submucosa and the lamina propria. Muscularis propria is a multilayered smooth muscle sheet located between the submucosa and the serosa and consists of two smooth muscle layers: an inner circular SML (IC-SML) and an outer longitudinal SML (OL-SML) (Fig. 1). The SML of the intestine plays essential role in intestinal villus formation and mucosal movement, intestinal motility, and absorption.
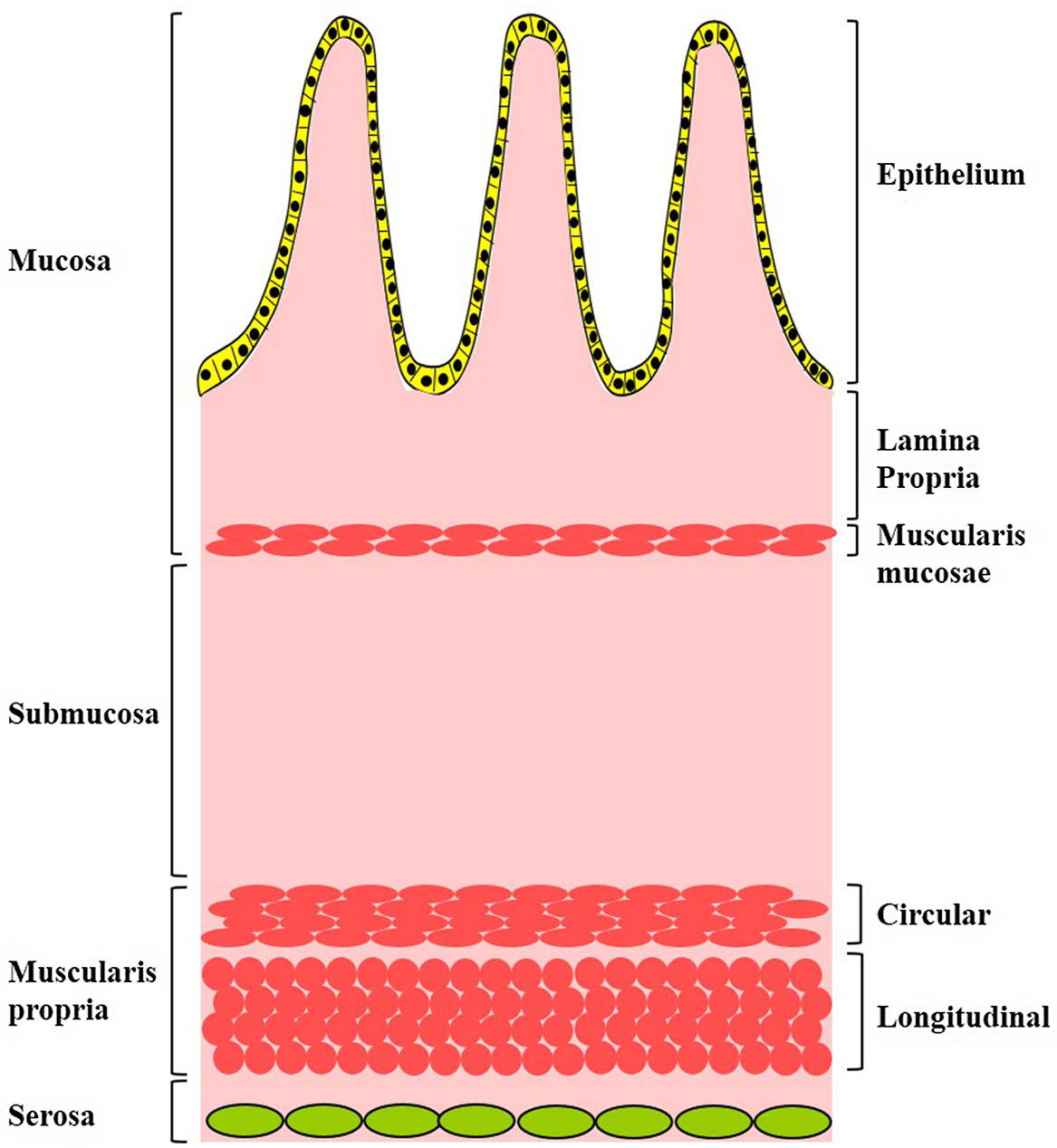
Fig. 1. Schematic diagram depicting the structures and the smooth muscle layers of the intestine wall.
Intestinal SML also contribute to the formation of sphincteric smooth muscle at specialized regions along the gastrointestine such as lower esophageal sphincter, pyloric sphincter, ileocecocolic sphincter, and internal or longitudinal anal sphincter. Our previous studies have revealed that anorectal malformations (ARMs) present defects of both the internal and the longitudinal anal sphincter,Reference Li, Ren and Xiao5,Reference Li, Diao, Ren and Liu6 but the relationship between dysregulated development of the intestinal SML and sphincter defects in ARMs is largely unknown. Reciprocal interactions between the intestinal tract mesenchyme and primitive endoderm is essential for the normal differentiation and maturation of both the intestinal SML and the intestinal epithelium.Reference Kedinger, Duluc, Fritsch, Lorentz, Plateroti and Freund7 Therefore, a detailed spatial and temporal developmental pattern of the intestinal SML in normal human embryos/fetuses is essential for the understanding of patho-mechanism underlying ARMs.
At the early phase of intestinal tract development, the normal intestinal tract contains many vacuoles,Reference Ferenc, Pietrzak and Godlewski8 and our recent study has demonstrated that the vacuoles fuse, expand, and develop into the mucosal epithelium during the process of gut recanalization at embryonic 6–7 week.Reference Liu, Song and Hao9 The reciprocal interactions of intestinal mucosal epithelium and the underlying intestinal mesenchyme induces the development of the SML of the intestine.Reference Powell, Pinchuk, Saada, Chen and Mifflin10–Reference McLin, Henning and Jamrich13 We have previously shown that that longitudinal and circular SMLs appear at the same time and their differentiation follows a rostro-caudal manner in human developing gut between week 7 and week 9.Reference Fu, Tam, Sham and Lui14 However, the temporal sequence of the formation of the longitudinal and circular SMLs beyond week 7 has not been determined.
This study aims to investigate the temporal and spatial pattern of the formation of the three intestinal SML via histological analysis of the developing intestine tract in human embryos and fetuses from 6 to 12 weeks of gestation. In addition, we also studied and compared the morphology of SML of neonates and embryos/fetuses.
Materials and methods
Materials and reagents
Phosphate buffer saline (PBS) solution, 4% of paraformaldehyde (PFA), ethanol, and sodium chloride were purchased from Sigma-Aldrich Ltd (St. Louis, MO). Hematoxylin and eosin (H&E) solution was purchased from Sigma-Aldrich Ltd. (St. Louis, MO). Primary monoclonal rabbit-anti-human alpha smooth muscle actin (α-SMA) antibody was purchased from Abcam (Cat No: ab124964. Cambridge, UK). Light microscope and digital camera (Olympus SZX7, Nikon, Eclipse E600, Japan) were used for tissue morphology.
Embryo and fetal tissues
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in Edinburgh 2000). Aborted human embryos/fetuses (6–12 weeks of gestation: 3 of crown-rump length (CRL) 8.5–9 mm, approximately gestation 6–7 weeks; 2 of CRL 28 mm, approximately gestation 9 weeks; 3 of CRL 31–36 mm, approximately gestation 10 weeks; 2 of CRL 72–84 mm, approximately gestation 11–12 weeks) were paraffin-embedded. Serial sagittal, horizontal and frontal sections (5 µm in thickness) were prepared. Hematoxylin and eosin (H&E) stained sections were scanned using high-quality digital scanner (3D HISTECH, Hungary). The use of human tissues for the study has been approved by the Medical Ethics Committee of Wuxi School of Medicine, Jiangnan University (Ethics approval number: JNU20190318IRB62).
Patient specimen collection
For the specimen collection of neonatal normal intestinal tract, two patients (1–3 days old) with intestinal obstruction (due to mesenteric hiatal hernia and fibrous remnant of omphaloenteric duct) undertook small bowel resection and anastomosis at the Department of Surgery of Capital Institute of Pediatrics affiliated Children Hospital (Beijing, China) between November 2019 and February 2020. The normal intestinal wall tissues around the incision were harvested and used for the study. The use of human intestine tissues for the study was approved by the Medical Ethics Committee of Capital Institute of Pediatrics affiliated Children Hospital (Beijing, China). Written informed consents were obtained from patients’ parents.
Immunohistochemistry
For immunohistochemistry staining of specimen, endogenous peroxidase was quenched by 3% hydrogen peroxide/methanol after sections were deparaffinized and rehydrated. After antigen retrieval in citrate buffer, sections were incubated at room temperature with blocking solution containing 5% normal goat serum (Dako Bioresearch, USA). The sections were incubated overnight in solution containing primary antibody (Dilution: 1:300) before rinsing in PBS solution and incubated with HRP-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Positive signals were developed using DAB (3, 3′-diaminobenzidine) and counterstained in hematoxylin solution. Sections were dehydrated in alcohol, cleared in xylene, and mounted in neutral balsam. Sections were examined under light microscope (Olympus BX40).
Masson trichrome staining
Masson trichrome staining was performed following our previous reportsReference Zhang, Liu, Wang, Peng and Wong15–Reference Liu, Gao, Du, Zhao and Wong17. In brief, the rehydrated sections were stained in preheated Bouin’s solution at 56°C for 15 min, followed by washing in tap water to remove extra dye. Sections were immersed in working Weigert’s iron hematoxylin solution for 5 min followed by rinsing in water. Sections were further stained in Biebrich scarlet-acid Fucshin solution, then in phosphotungstic/phosphomolybdic acid solution, and finally in Aniline Blue solution (5 min each), followed by a 2 min staining in acetic acid (1%) and rinsing in tap water. Sections were dehydrated in alcohol, cleared in xylene, and mounted in neutral balsam. Sections were examined under light microscope (Olympus BX40).
Histology analysis
The normal intestinal wall tissue after birth was fixed in 10% formalin (24 h at 4°C), before being dehydrated in alcohol, cleared in xylene, and embedded in paraffin. Sections (5 μm thick) were prepared and mounted onto TESPA-coated microscopic glass. Sections were dewaxed, rehydrated for H&E staining (Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 10 min followed by differentiation in a hydrochloride acid-alcohol mixture, and counterstaining in eosin solution for 3 min. Sections were dehydrated in ethanol, cleared in xylene, and mounted in neutral balsam before examination under light microscope (Olympus BX40).
Results
No SML is formed in the developing gut of 6–7 weeks embryos
To investigate the development of the intestinal SML, we performed histology and immunohistochemistry of human embryos/fetuses of 6–12 weeks of gestation. The intestine of embryos of 6–7th week of gestation has no lumen (Fig. 2A and 2B), and the primitive intestine was filled with mesenchymal cells and vacuoles covered by a monolayer of epithelial cells (Yellow triangle, Fig. 2C and 2D). The mucosa, the submucosa, and the muscularis propria including the inner circular muscle and the outer longitudinal muscle were not discernible at this period. Lack of lumen in the intestine of embryos of 6–7th week of gestation is in line with the observation that the intestinal lumen only starts to develop at the 8th week of gestation.Reference Liu, Song and Hao9

Fig. 2. Histology study of 6–7th week of gestation human embryos. H&E staining of sagittal sections of human embryos of CRL 8.5–9 mm (approximately gestation 6–7 weeks). Regions highlighted were labeled and magnified as shown. (A) A lateral sagittal section. (B) Magnified view of Panel B. (C) Magnified view of Panel C. (D) Magnified view of Panel D.
Identification of intestinal IC-SML in embryo and fetus of 8–9 weeks of gestation
We next examined embryos of 8–9 weeks of gestation. The liver occupied much of the peritoneal cavity and the physiological hernia pushed part of the primitive intestine out of the peritoneum through the primitive umbilicus (Fig. 3A and 3B). Histology analysis showed a marked morphological difference as compared with embryos of 6–7th week of gestation, in that a narrow continuous gut lumen (incomplete recanalization) was now identifiable (compare Fig. 2B with Fig. 3B), and vacuoles were still seen in gut lumen (asterisk in Fig. 3C and 3D), suggesting that the embryonic gut was at the early stage of intestine recanalization. The mucosal epithelium was clearly demarcated in the 8–9 weeks embryonic intestine. Meanwhile, the SML could be visualized (filled arrows, Fig. 3C, 3D and 3E), and circular muscle fibers were observed under a higher magnification (filled arrows, Fig. 3c1, 3d1, 3e1). Later at the 9th week of gestation, part of the primitive intestine was still located out of the peritoneal wall (Fig. 4a), a few of vacuoles were still found in the gut lumen (asterisk, Figs. 4B, 4C and 3D). Mucosal epithelium (unfilled arrows) and SML were clearly seen (filled arrows, Figs. 4B, 4C and 3D). Circular muscle fibers were also identifiable (arrows, Fig. 4B, 4C and 4D).

Fig. 3. Histology study of 9th week of gestation human fetuses. H&E staining of sagittal sections of human fetuses of CRL 22 mm (approximately gestation 9 weeks). Regions highlighted were labeled and magnified as shown. (A) A lateral sagittal section. (B) Magnified view of Panel B. (C, D, and E) Magnified view of Panels C, D, and E, respectively. (C) Vacuoles (asterisk) adjacent to the intestine wall appeared merging with the developing mucosal epithelium, and the SML (Filled arrows) could be seen. (c1, d1 and e1) The inner circular SML (Filled arrows) were developed in the intestine wall. Intestinal epithelium was indicated with unfilled arrow.

Fig. 4. Histology study of 10th week of gestation human fetuses. H&E staining of sagittal sections of human fetuses of CRL 35 mm (approximately gestation 10 weeks). Regions highlighted were labeled and magnified as shown. (A) A lateral sagittal section. (B, C, and D) Magnified view of Panels B, C, and D, respectively. Vacuoles (asterisk) adjacent to the intestine wall appeared merging with the developing mucosal epithelium (unfilled arrows). The SML (Filled arrows) were seen. (c1, d1, and e1) the inner circular SML (Filled arrows) were clearly seen in the intestine wall.
OL-SML of intestinal wall formed later than IC-SML
At the 10th week of gestation, histology analysis showed that the primitive intestine has further developed, in that a relatively big and prominent luminal space could be seen with very few vacuoles (asterisk, Fig. 5A and 5B), indicating that the primitive intestine was at the later stage of intestinal recanalization. Two distinct SML were now discernible in the intestinal wall (Fig. 5C and 5D), with circular muscle fiber (filled arrow) at the inner and longitudinal muscle fiber (arrowheads) at the outer perimeter of the intestine (Fig. 5c and 5d), indicating that OL-SML has developed in the primitive intestine. Immunostaining for smooth muscle alpha actin (α-SMA), a marker for intestinal smooth muscle, was performed on adjacent sections and showed that both the circular muscle layer (filled arrow) and the longitudinal muscle layer (arrowheads) were immunopositive for α-SMA (Fig. 5e and 5f), which confirmed the development of the OL-SML and the IC-SML at this embryonic stage. In contrast, no muscularis mucosae-like structure and no α-SMA-immunoreactivity could be identified underneath the mucosal epithelium, which indicated that muscularis mucosae was not formed in the intestine of the embryo at the 10th week of gestation (Fig. 5e and 5f).

Fig. 5. Histology study of 12th week of gestation human fetuses. H&E staining and immunohistochemistry of coronal sections of human fetuses of CRL 72 mm (approximately gestation 12 weeks). Regions highlighted were labeled and magnified as shown. Enlarged gut lumen could be seen in the developing intestine. Less vacuoles were noted in gut. (A) A coronal section. (B) Magnified view of Panel B. (C and D) Magnified view of Panels C and D. Vacuoles (asterisk) in the developing gut lumen. (c and d) In addition to the inner circular SML (Filled arrows), the outer longitudinal SML (arrowheads) could be clearly seen. (E and F) Serial sections were immunostained for α-SMA. (e and f) α-SMA expression (brown) was localized at the inner circular SML (Filled arrows) and the outer longitudinal SML (arrowheads).
Identification of muscularis mucosae in the intestine in embryo and fetus of 11–12 weeks of gestation
Later at the 11–12th week of gestation, H&E staining and Masson trichrome staining showed that, in addition to the IC-SML (filled arrow) and OL-SML (arrowheads), cluster of cells was found to aggregate forming a muscular-like layer (yellow arrows) between the mucosa and submucosa (Fig. 6a and 6b). Immunostaining showed that this muscular-like layer was immunopositive for α-SMA (Fig. 6C, 6c and 6c1), which indicated that the muscularis mucosae had started to differentiate at this embryonic stage.

Fig. 6. Histology study of 13th week of gestation human fetuses. H&E, Masson Trichrome staining and immunostaining for α-SMA of transverse sections of human fetuses of CRL 84 mm (approximately gestation 13 weeks). Regions highlighted were labeled and magnified as shown. (A) H&E staining; (B) Masson Trichrome staining; (C) immunostaining for α-SMA. (a, b, and c) Magnified view of region a, b, and c to show the rectal wall. (a1 and b1) The inner circular SML (Filled arrows) and the outer longitudinal SML (arrowheads) could be seen. Yellow arrowheads indicated the putative muscularis mucosae. (c1) Immunohistochemistry to localise α-SMA expression in the gut wall. Immunoreactivity for α-SMA (brown) beneath the epithelium indicated the development of muscularis mucosae (arrows). The inner circular SML (unfilled arrows) and the outer longitudinal SML (arrowheads) were well formed.
Taken together all the above, our study revealed that in the human developing gut, IC-SML developed first, followed by OL-SML, and muscularis mucosae developed last between week-8 to week-12 of gestation.
SML in neonatal intestinal tract are morphologically different from that in fetal intestine
H&E staining and Masson Trichrome staining of the neonatal 1–3 days old normal intestinal wall showed the three layers of smooth muscle (Fig. 7A and 7B). The muscularis mucosae was localized beneath the epithelium (Fig. 7a1 and 7b1). The IC-SML and the OL-SML were well formed and thick (Fig. 7a2 and 7b2) with clear circular or longitudinal muscle fibers. Immunohistochemical staining showed all the three SML expressed high level of α-SMA (Fig. 7C). The smooth muscles in lamina propria are required for peristalsis of epithelium in gastrointestinal tract.Reference Faussone-Pellegrini18 Immunoreactivity for α-SMA was also detectable at the narrow space of the lamina propria, with branching into the glands of the lamina propria in mucosa (Fig. 7c1), further suggesting the lamina propria in mucosa had histological structure involving in peristalsis of epithelium at early phase of neonatal period. The morphology and the α-SMA staining pattern of the IC-SML and the OL-SML in neonatal intestine were different from to that in fetuses of 10–12th week of gestation (Compared Figs. 5, 6 and 7). Compared with the fetuses of 10–12th week of gestation, the muscularis mucosae in neonatal intestinal tract became more compact with branching into the glands of the lamina propria in mucosa, IC-SML and IC-SML were thicker with detectable well-differentiated smooth muscular fiber. Taken all these suggested that the three layers of intestinal SML were completely formed at around 12-week of gestation, and these SML have further developed and matured from 12-week of gestation onward into neonatal period.

Fig. 7. Histology study of the normal neonatal jejunal wall. H&E, Masson Trichrome staining and immuno staining for α-SMA of serial transverse sections of the normal jejunal wall. Regions highlighted were labeled and magnified as shown. (A) H&E staining; (B) Masson Trichrome staining; (C) immunohistochemistry for α-SMA. (a1 and b1) Muscularis mucosae (arrow); (c1) immunoreactivity for α-SMA (brown) beneath the epithelium indicated the development of muscularis mucosae (arrows); (a2 and b2) the outer longitudinal SML (arrowheads) and the inner circular SML (arrows) under H&E and Masson Trichrome staining; (c2) immunoreactivity for α-SMA (brown) at the outer longitudinal SML (triangle) and the inner circular SML (arrowhead).
Discussion
Intestinal smooth muscle develops from a continuous layer of amorphous mesenchyme positioned between a primitive epithelium (presumptive mucosa) on the luminal side and primitive mesothelium (presumptive visceral peritoneum) on the serosal side.Reference McLin, Henning and Jamrich13,Reference Faussone-Pellegrini18,Reference Tatekawa, Yamanaka, Hasegawa and Sonobe19 Studies on mouse embryos have indicated that the intestinal smooth muscle developed in a cranial to caudal fashion, and the inner circular muscle layer is formed earlier than the outer longitudinal muscular layer in mouse embryos.Reference Yamada, Udagawa and Matsumoto20–Reference Jahan, Rafiq, Matsumoto, Jahan and Otani22 However, the temporal and spatial pattern of the formation of the inner circular muscle layer (IC-SML), the outer longitudinal muscular layer (OL-SML), and the muscularis mucosae of the intestine in human embryos and fetuses is not known. Our study revealed that in the human developing gut from week-8 to week-12 of gestation, the IC-SML developed first, followed by the OL-SML and the muscularis mucosae developed last. Current finding that SMA-immunoreactivity was detected at the IC-SML and OL-SML regions, but no detectable SMA-immunoreactivity at the mucosae of week-12 embryonic gut corroborated with our previous finding that muscularis muscosae developed later than the IC-SML and OL-SML.Reference Fu, Tam, Sham and Lui14 In addition, our data also showed that the intestine of embryos of 6–7th week of gestation was filled with mesenchymal cells and vacuoles covered by a monolayer of epithelial cells with no intestinal lumen, no discernible mucosa, submucosa, muscularis propria including the inner circular muscle and the outer longitudinal muscle. In contrast, our previous report that that both the longitudinal and circular SMLs already formed at the week 7 human embryonic gut.Reference Fu, Tam, Sham and Lui14 Current study included samples of embryonic gut spanning from the stage of gut lumen formation to the formation of the SMLs and the muscularis mucosae and showed that the IC-SML developed first, followed by the OL-SML, and the muscularis mucosae developed last. The differences between the current findings and our previous reportReference Fu, Tam, Sham and Lui14 are likely attributable to the discrepancies in embryo staging of our previous report. Furthermore, our study also revealed that the muscularis mucosae, IC-SML and OL-SML of embryonic/fetal gut and neonatal gut displayed different morphology, which suggests that the three SML develops and matures from 12-week of gestation onwards into neonatal period.
The intestinal tract development is a complex process that requires precise interaction of distinct types of cells derived from all the three embryonic germ layers: epithelial cells arise from endoderm, smooth muscle cells and fibroblasts arise from mesoderm, and intrinsic neurons arise from ectoderm. The development of intestinal tract requires the proper epithelial-to-mesenchymal cross talks and various molecular signalings, whereas miscues in these processes will cause deformation and diseases associated with the intestinal tract.Reference McHugh23 In our previous study, we investigated the morphological differences on the anorectal muscles of normal neonate cadaveric and patients with anorectal malformation relevant disease and discovered that the internal anal sphincter was absent and the longitudinal sphincter only arisen from the levator ani in patients with anorectal malformation.Reference Li, Ren and Xiao5,Reference Li, Diao, Ren and Liu6 The internal anal sphincter and the longitudinal anal sphincter are derived from IC-SML and OL-SML of the rectum, respectively.Reference Muranaka, Nakajima and Iwaya24,Reference Keef and Cobine25 The absence of the internal anal sphincter and longitudinal anal sphincter in patients with anorectal malformation could be caused by the abnormal development of IC-SML/OL-SML in the rectum.
Conclusion
Present study reported for the first time the temporal pattern of development of the three SML in human embryos and fetus from week-8 to week-12 of gestation, and the morphological changes of the three SML between human fetuses and neonates. Perturbation of the developmental events of the intestinal SML could contribute to developmental malformations including anorectal malformation of gut in humans.
Acknowledgments
The authors would like to thank all the parents and patients who participated in this study. This work is supported by the Beijing Municipal Administration of Hospitals (XTZD20180302).
Financial Support
The authors have declared no financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest
The authors have declared no conflicts of interest.
Ethical standards
Collection of human specimens experiment involving human tissues were performed with ethical approvals from relevant regulatory bodies.










