Iodine is essential for the production of thyroid hormones, which are required for the normal growth and neurocognitive development of the fetus, infant and child( Reference Melse-Boonstra and Jaiswal 1 ). Because pregnancy is characterized by an increased demand for thyroid hormones, the iodine requirement of pregnant women is more than two-thirds higher than that of non-pregnant women( Reference Li and Eastman 2 ). Severe iodine deficiency during pregnancy can lead to miscarriage, stillbirth, congenital anomalies, perinatal mortality and cretinism in the offspring( Reference Hetzel 3 ). Mild-to-moderate iodine deficiency in pregnancy, particularly during the first trimester, has also been associated with cognitive deficits and neurobehavioural disorders in the offspring( Reference Vermiglio, Lo Presti and Moleti 4 , Reference van Mil, Tiemeier and Bongers-Schokking 5 ).
Historically, iodine deficiency has primarily been considered a concern for developing countries; however, suboptimal iodine status among pregnant women has been reported recently in developed countries including Australia, New Zealand, the UK, Ireland, the USA, France, Spain and Hong Kong( Reference Li and Eastman 2 ). The WHO recommends universal salt iodization – the iodization of all salt for human and livestock consumption – as the optimal strategy for the prevention of iodine deficiency( Reference Andersson and de Benoist 6 ). In addition, in areas where the proportion of households using iodized salt has yet to reach 90 %, the WHO recommends iodine supplementation be made available to all women capable of reproduction, currently pregnant or lactating( Reference Andersson and de Benoist 6 ).
In response to the re-emergence of iodine deficiency in Australia and New Zealand, the joint governmental agency, Food Standards Australia New Zealand, mandated the replacement of non-iodized salt in all non-organic, yeast-leavened bread with iodized salt in late 2009( 7 ). As it was understood that the iodine requirements of pregnant and breast-feeding women remained unmet following this mandate, an iodine supplement recommendation of 150 μg/d throughout pregnancy and lactation was issued in both Australia and New Zealand in mid-2010( 8 ). Iodine supplements were made available on prescription in New Zealand for a subsidized price ($NZ 3) and some major Australasian supplement manufacturers reformulated their prenatal/lactation products to comply with the new recommendation; however, no formal public health educational campaign was initiated in New Zealand.
The impact of bread fortification on the iodine intakes of New Zealand women before and during pregnancy has not yet been estimated, nor has the uptake of recommended maternal iodine supplementation. Given the disparities noted worldwide in the recommended uptake of folic acid supplements for pregnancy( Reference Ray, Singh and Burrows 9 ), sociodemographic position may also be a particularly important factor in the uptake of iodine supplements. The aims of the present study were therefore to assess the impact of these two recently introduced public health policies on the estimated iodine intakes of women before and during pregnancy and to evaluate whether all segments of this target population have benefited equally from the maternal iodine supplement recommendation.
Methods
Population
The Vitamins and Minerals in Pregnancy Survey was a survey of postpartum New Zealand women between 7 March and 15 April, 2011. Twelve maternity wards/hospitals, ten sites in New Zealand's six largest cities and two sites in smaller centres, were invited to participate based on their affiliation with the University of Otago. Eleven sites agreed to participate, including: Tauranga Hospital, Tauranga; Whakatane Hospital, Whakatane; River Ridge East Birth Centre, Waterford Birth Centre and Waikato Hospital, Hamilton; Hutt Hospital, Lower Hutt; Kenepuru Community Hospital and Wellington Hospital, Wellington; Christchurch Women's Hospital, Christchurch; Queen Mary Maternity Hospital, Dunedin; and Southland Hospital, Invercargill. Women were eligible for inclusion when they had delivered a healthy term infant, were aged 18 years or over and could communicate in English. Ethical approval for the study was obtained from the Multi-Region Ethics Committee of the New Zealand Ministry of Health (reference: MEC/11/EXP/004).
Questionnaire
Dietary supplement use, bread intake and iodized salt use before and during pregnancy were assessed using an anonymous, self-administered questionnaire developed with reference to similar questionnaires used elsewhere( Reference Dott, Rasmussen and Hogue 10 , Reference Charlton, Gemming and Yeatman 11 ). Questions on vitamin and mineral knowledge and maternal sociodemographic and obstetric characteristics were also included. Pre-testing and refinement of the questionnaire were undertaken with pregnant volunteers.
Iodine intake from bread
Usual daily intake of bread before pregnancy and during each trimester of pregnancy was queried. Participants were asked whether they ate non-organic, shop-bought bread. If yes, participants were asked how many slices of bread they consumed per day in the month before pregnancy and during months 1–3, 4–6 and 7–9 of pregnancy. Using the analytically determined post-fortification level of 43 μg iodine/100 g bread (range: 32–53 μg iodine/100 g bread)( Reference Edmonds and Ryan 12 ), one slice of bread with a median weight of 33 g( Reference Lesperance 13 ) was estimated to provide 14 μg of iodine, with items such as rolls and buns being equivalent to two bread slices.
Iodine intake from supplements
A full-colour show card depicting photographs of all maternal supplements purchasable within New Zealand accompanied the questionnaire. Supplemental iodine intake for each individual was calculated based on reported supplement brand(s), if any, frequency of use (from twice daily to less than once weekly) and period of use (month before pregnancy, months 1, 2, and 3 of pregnancy and during trimesters 2 and 3 of pregnancy). Immediately following this survey, a major pregnancy supplement manufacturer reformulated its supplement to include iodine (250 μg/tablet), and thus all estimates here are based on the reformulated value to better assess the true impact of iodine supplementation in the New Zealand pregnant population.
Iodine intake from food and salt
Each participant was assigned an estimated baseline iodine intake from food of 60 μg/d. This was the mean intake of women determined prior to the iodine fortification of bread in the 2003–2004 New Zealand Total Diet Survey, based on 14 d simulated diets( Reference Thomson, Vannoort and Haslemore 14 ). Iodized salt use was excluded from the Total Diet Survey estimate. In our questionnaire, salt use before and during pregnancy was queried, with options including: iodized, non-iodized/none and unsure. Participants who used iodized salt before or during pregnancy were assigned a further 48 μg iodine/d, based on an estimated intake of 1 g discretionary salt/d( 7 ) and an analytically determined median of 48 mg iodine/kg iodized salt( Reference Thomson 15 ).
Statistical analysis
All analyses were conducted using the statistical software package Stata 11·1 and a two-sided 0·05 level of significance was used in all cases. Participants who nominated two or more ethnic groups were assigned to a single ethnic group using the prioritization system recommended by Statistics New Zealand( 16 ). Proportional to recent national maternity data( 17 ), under-represented age–ethnicity subgroups were weighted up and over-represented subgroups were weighted down to ensure estimates were representative in terms of age and ethnicity. All analyses incorporated post-stratification weights and included sites as clusters to estimate robust standard errors. Mixed-model regression analyses were performed to estimate mean intakes and 95 % confidence intervals of iodine from fortified bread, before and during pregnancy, with sites treated as random effects. Independent variables included: maternal age (three categories), parity (four categories), ethnicity (five categories), education (five categories), household income (five categories), relationship status (cohabiting v. not) and pregnancy intention (planned v. unplanned). Similarly, mixed-model regression analyses were used to estimate the proportion of women with mean iodine intakes from supplements below the New Zealand Ministry of Health recommendation for pregnancy (150 μg/d)( 8 ) and to estimate the proportion and 95 % confidence interval of women with mean iodine intakes below the Estimated Average Requirement (EAR) for iodine before and during pregnancy (107 μg iodine/d and 179 μg iodine/d, respectively). The proportion of a population with nutrient intakes beneath the EAR can be used to estimate the prevalence of inadequate intakes( Reference Allen, de Benoist and Dary 18 ). The EAR were derived from the WHO Recommended Nutrient Intakes for non-pregnant, non-lactating women (150 μg iodine/d) and pregnant women (250 μg iodine/d), using a conversion factor of 1·4 as recommended by the WHO and FAO( Reference Allen, de Benoist and Dary 18 ). Missing values for iodine intake from bread and supplements were treated as follows. Where a value was missing, the known bread or supplement value, the baseline intake (60 μg/d) and the intake from iodized salt (48 μg/d or none) were summed. If this did not reach the EAR cut-off, the total intake value was classified as missing. In the case of missing data on iodized salt use, or if a participant had selected ‘unsure’ for iodized salt use, where the total intake value would not have reached the specified cut-off had the participant used iodized salt (i.e. the partial total was more than 48 μg/d below the cut-off), the individual was classified as having not met the cut-off. In all cases where the partial total reached or exceeded the EAR cut-off, the individual was classified as having met that cut-off.
Results
Study sample characteristics
Of the 968 women invited to participate, 723 (75 %) agreed and met all inclusion criteria. Median maternal age was 31 years, similar to the national median recorded in the year ended March 2011 (30 years)( 19 ). Almost half of all deliveries were to primiparous women (45 %) and 44 % of all pregnancies were unplanned. Two-thirds (66 %) of women held a post-secondary qualification, and 42 % reported an annual household income below the national median for 2010 ($NZ 64 272)( 20 ). Compared with recent national maternity data, this sample had a higher proportion of New Zealand Europeans (65 % v. 55 %), a lower proportion of Māori (14 % v. 20 %) and Pacific women (5 % v. 11 %), and a similar proportion of Asians (9 % v. 11 %)( 17 ).
Effect of bread fortification on estimated iodine intakes of women prior to pregnancy
Prior to pregnancy, 20 % of women reported that they did not use iodized salt and a further 7 % were unsure of what type of salt they used. On average, fortified bread provided 37 μg iodine/d before pregnancy (Table 1). Younger women, women with greater parity, Māori and Pacific women, women with the least income and education, single women and women with unplanned pregnancies attained statistically significantly higher mean iodine intakes from bread than their counterparts (all P ≤ 0·043). An estimated 22 % of those surveyed would have fallen below the EAR for non-pregnant, non-lactating women without the iodine fortification of bread (Table 2). Including iodine intake from fortified bread, the overall proportion of non-pregnant women falling below the EAR was reduced to 16 %. The least advantaged women benefited the most from the mandatory fortification of bread, with a reduction of up to two-thirds in the proportion falling below the EAR in some groups.
Table 1 Mean iodine intakes from bread fortified with iodized saltFootnote * before and during pregnancy: postpartum New Zealand (NZ) women, Vitamins and Minerals in Pregnancy Survey, 2011
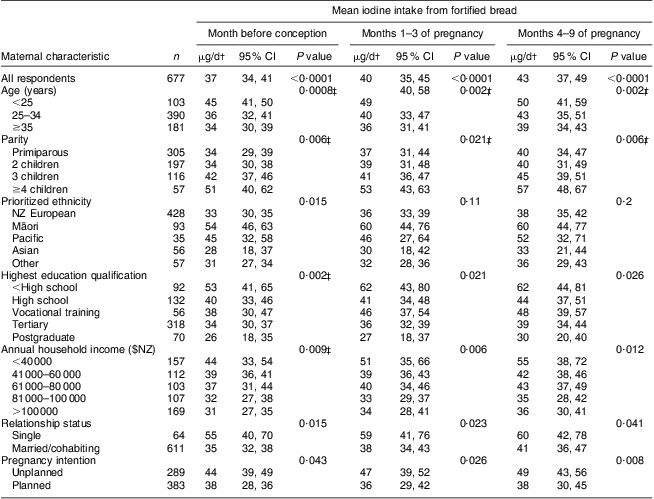
* Estimated iodine intake from iodine-fortified bread = 14 μg/slice (43 μg iodine/100 g bread, 1 slice = 33 g)( Reference Edmonds and Ryan 12 , Reference Lesperance 13 ).
† Weighted by age and ethnicity and adjusted for clustering by site.
‡ P value for linear trend.
Table 2 Estimated proportion of women before conception with iodine intakes from foodFootnote * below the Estimated Average Requirement (EAR; 107 μg/d) as derived from WHO's Recommended Nutrient Intake (150 μg/d) for non-pregnant, non-lactating women( Reference Allen, de Benoist and Dary 18 ): postpartum New Zealand (NZ) women, Vitamins and Minerals in Pregnancy Survey, 2011
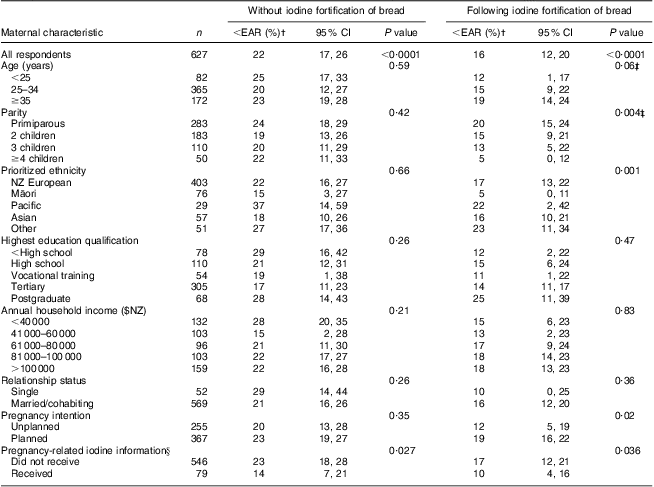
* Median iodine intake from foods (excluding iodine-fortified bread and iodized salt) = 60 μg/d( Reference Thomson, Vannoort and Haslemore 14 ); estimated iodine intake from iodized salt, if used = 48 μg/d (1 g salt/d iodized at 48 mg/kg salt)( 7 , Reference Thomson 15 ); estimated iodine intake from iodine-fortified bread = 14 μg/slice (43 μg iodine/100 g bread, 1 slice = 33 g)( Reference Edmonds and Ryan 12 , Reference Lesperance 13 ).
† Weighted by age and ethnicity and adjusted for clustering by site.
‡ P value for linear trend.
§ Information received prior to pregnancy only.
Combined effect of supplement recommendation and bread fortification on estimated iodine intakes before and during pregnancy
Overall, 16 % of all women and 28 % of women with planned pregnancies took supplements containing iodine in the month before conception, the majority (>90 %) of which were commercial prenatal supplements. Including intake from food, iodized salt, fortified bread and supplements, an estimated 13 % of women failed to attain the EAR in the month before conception.
In the first month of pregnancy, over two-thirds of women did not meet the pregnancy EAR (Table 3). By the third month of pregnancy, this had decreased to approximately half of all women, and remained at this level during months 4–9. When estimated excluding the iodine contributed by the reformulated commercial supplement, the proportion below the EAR was considerably greater at 84 % in month 1, 76 % in month 2, 71 % in month 3 and 62 % in months 4–9 (data not shown).
Table 3 Estimated proportion of women during pregnancy with iodine intakes below the Estimated Average Requirement (EAR; 179 μg/d) as derived from the WHO's Recommended Nutrient Intake for pregnancy (250 μg/d)( Reference Allen, de Benoist and Dary 18 ), following the iodine fortification of bread and including supplement useFootnote *: postpartum New Zealand (NZ) women, Vitamins and Minerals in Pregnancy Survey, 2011

* Median iodine intake from foods (excluding iodine-fortified bread and iodized salt) = 60 μg/d( Reference Thomson, Vannoort and Haslemore 14 ); estimated iodine intake from iodized salt, if used = 48 μg/d (1 g salt/d iodized at 48 mg/kg salt)( 7 , Reference Thomson 15 ); estimated iodine intake from iodine-fortified bread = 14 μg/slice (43 μg iodine/100 g bread, 1 slice = 33 g)( Reference Edmonds and Ryan 12 , Reference Lesperance 13 ).
† Weighted by age and ethnicity and adjusted for clustering by site.
‡ P value for linear trend.
Younger women, women with higher parity, single women and those with unplanned pregnancies were more likely to have mean iodine intakes beneath the EAR throughout pregnancy (all P ≤ 0·022). Women with less education and income, and those who had not received pregnancy-related iodine information, were also less likely to meet the EAR; however, this was not statistically significant for all months. Pairwise comparisons revealed that indigenous Māori women were less likely than New Zealand Europeans to meet the EAR in months 2, 3 and 4–9 of pregnancy (all P ≤ 0·041, data not shown). Similarly, Pacific women were less likely than New Zealand Europeans to meet the EAR in months 1, 2 and 3 of pregnancy (all P ≤ 0·007, data not shown).
Supplement uptake by sociodemographic subgroup
Across the first three months of pregnancy, uptake of iodine supplementation at or above the New Zealand Ministry of Health's recommended level for pregnancy (150 μg/d)( 8 ) increased progressively (Fig. 1(a) to Fig. 1(f)). However, uptake was not uniform across sociodemographic subgroups, with the least advantaged women being less likely to take supplements as recommended. Twenty-two per cent of women attained the recommended iodine intake from supplements in the first month of pregnancy. This proportion increased to 33 % in the second month, 39 % in the third month, and then declined to 35 % in trimesters 2 and 3. From the second month of pregnancy, having received pregnancy-related iodine information approximately doubled the likelihood of taking iodine supplements as recommended, and those who received information also maintained high rates of uptake in the second and third trimesters. Less than half (49 %) of all women received pregnancy-related iodine information before or during pregnancy.
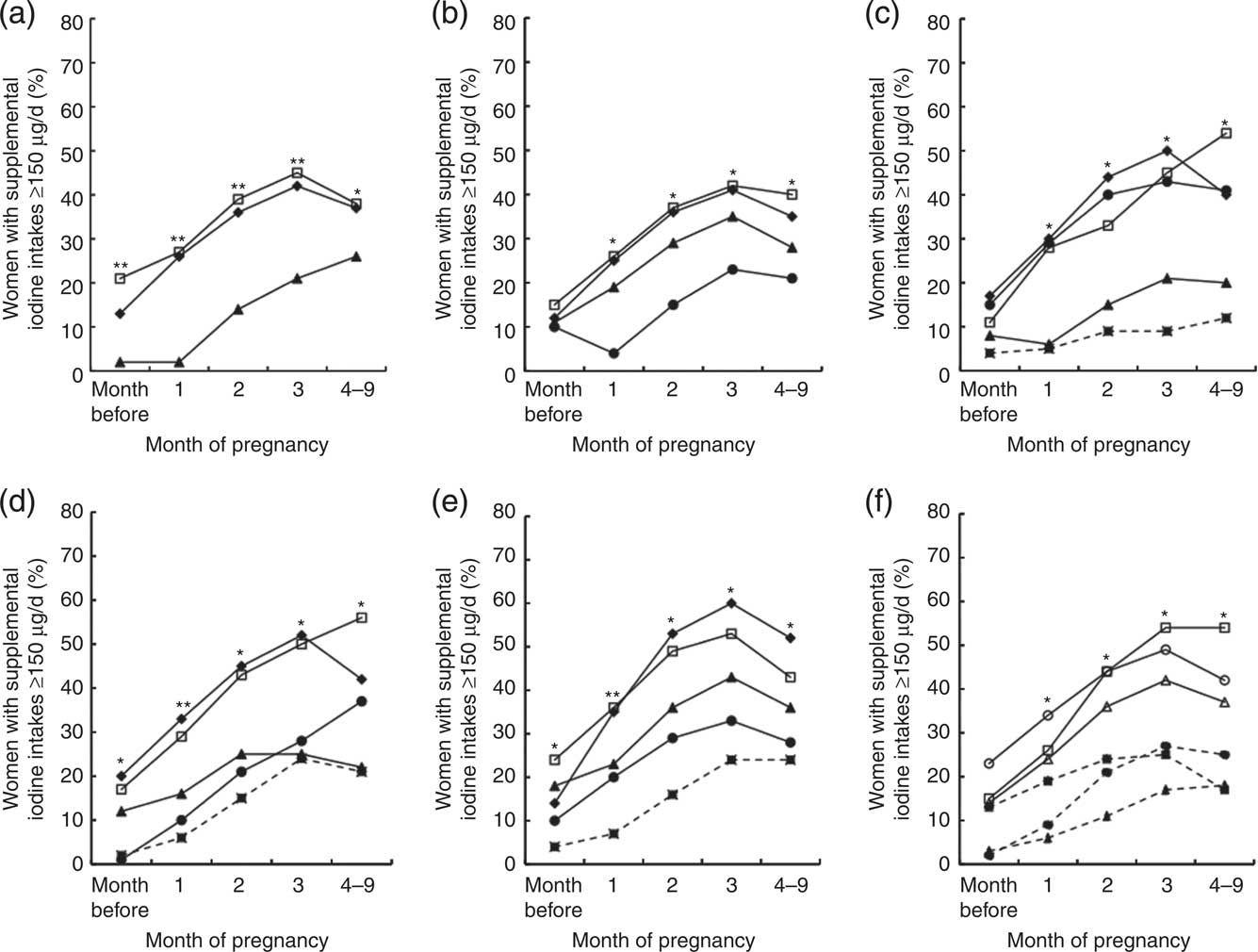
Fig. 1 Proportion (%) of postpartum New Zealand women with supplemental iodine intakes ≥150 μg/d according to: (a) age at delivery (
![]() $$$$
, <25 years;
$$$$
, <25 years;
![]() $$$$
, 25–34 years;
$$$$
, 25–34 years;
![]() $$$$
, ≥35 years); (b) parity (
$$$$
, ≥35 years); (b) parity (
![]() $$$$
, primiparous;
$$$$
, primiparous;
![]() $$$$
, 2 children;
$$$$
, 2 children;
![]() $$$$
, 3 children;
$$$$
, 3 children;
![]() $$$$
, ≥4 children); (c) prioritized ethnicity (
$$$$
, ≥4 children); (c) prioritized ethnicity (
![]() $$$$
, New Zealand European;
$$$$
, New Zealand European;
![]() $$$$
, Māori;
$$$$
, Māori;
![]() $$$$
, Pacific;
$$$$
, Pacific;
![]() $$$$
, Asian;
$$$$
, Asian;
![]() $$$$
, Other); (d) highest education qualification (
$$$$
, Other); (d) highest education qualification (
![]() $$$$
, <high school;
$$$$
, <high school;
![]() $$$$
, high school;
$$$$
, high school;
![]() $$$$
, vocational;
$$$$
, vocational;
![]() $$$$
, tertiary;
$$$$
, tertiary;
![]() $$$$
, postgraduate); (e) annual household income (
$$$$
, postgraduate); (e) annual household income (
![]() $$$$
, <$NZ 40 000;
$$$$
, <$NZ 40 000;
![]() $$$$
, $NZ 41 000–60 000;
$$$$
, $NZ 41 000–60 000;
![]() $$$$
, $NZ 61 000–80 000;
$$$$
, $NZ 61 000–80 000;
![]() $$$$
, $NZ 81 000–100 000;
$$$$
, $NZ 81 000–100 000;
![]() $$$$
, >$NZ 100 000); and (f) receipt of pregnancy-related iodine information (
$$$$
, >$NZ 100 000); and (f) receipt of pregnancy-related iodine information (
![]() $$$$
, received iodine information;
$$$$
, received iodine information;
![]() $$$$
, did not receive iodine information), pregnancy intention (
$$$$
, did not receive iodine information), pregnancy intention (
![]() $$$$
, planned pregnancy;
$$$$
, planned pregnancy;
![]() $$$$
, unplanned pregnancy) and relationship status (
$$$$
, unplanned pregnancy) and relationship status (
![]() $$$$
, married/cohabiting;
$$$$
, married/cohabiting;
![]() $$$$
, single). Weighted for age and ethnicity and adjusted for clustering by site (n ≥ 660); Vitamins and Minerals in Pregnancy Survey, 2011. *P ≤ 0·05, **P ≤ 0·0001
$$$$
, single). Weighted for age and ethnicity and adjusted for clustering by site (n ≥ 660); Vitamins and Minerals in Pregnancy Survey, 2011. *P ≤ 0·05, **P ≤ 0·0001
Discussion
Given the implications for neurocognitive development, the decision to implement public health policies to redress iodine insufficiency in Australia and New Zealand was warranted and was in alignment with strategies advocated by the WHO. In doing so, our survey suggests that mandating the iodine fortification of bread has reduced the prevalence of inadequate iodine intakes among non-pregnant, non-lactating New Zealand women by almost one-third, although women with lower bread intakes, such as women of Asian ethnicity and those with a postgraduate education, did not benefit to the same degree. Despite subsidizing the cost, recommended iodine supplement uptake during pregnancy was non-uniform across sociodemographic subgroups, with the most disadvantaged benefiting the least from this public health strategy, mirroring patterns noted worldwide for folic acid supplement uptake( Reference Mallard, Gray and Houghton 21 ). With a decreased likelihood of reaching their full intellectual potential, existing sociodemographic disparities faced by children born to younger women, women with higher parity, Māori and Pacific women, women with less education and income and single women may thus be further exacerbated.
Few studies have examined the uptake of iodine supplement recommendations according to sociodemographic position. In a recent study in Poland, where prenatal iodine supplementation is also officially recommended, similar sociodemographic disparities were apparent in the uptake of iodine-containing supplements( Reference Milewicz, Czyzewicz and Stochmal 22 ). Several researchers have recently questioned the reliance upon iodine supplements as a means to provide coverage to all pregnant women, highlighting that official recommendations and associated educational campaigns may fail to benefit women of lower socio-economic position( Reference Zimmermann and Delange 23 , Reference Untoro, Timmer and Schultink 24 ). International evidence has shown that folic acid supplement campaigns meet with little success in lower socio-economic groups( Reference Ray, Singh and Burrows 9 ). Moreover, educational campaigns have been theorized to widen disparities by increasing supplement uptake among women receptive to such initiatives, but being unable to influence those least likely to use supplements( Reference Sumar and McLaren 25 ). Conversely, universal policies, such as mandatory food fortification, alter the underlying environment rather than attempt to modify behaviour and may therefore narrow inequalities in nutrient intakes( Reference Sumar and McLaren 25 ). The disparities in supplement uptake noted here, despite subsidizing the cost of supplements, thus highlight the need for prioritizing further efforts towards universal salt iodization, such as the mandatory fortification of additional processed foods with iodized salt.
In developed countries, more than three-quarters of salt intake is derived from processed foods( Reference James, Ralph and Sanchez-Castillo 26 , Reference Mattes and Donelly 27 ), somewhat limiting the effectiveness of salt iodization programmes unless food manufacturers use iodized salt. While voluntary use of iodized salt in processed foods is permitted in New Zealand, manufacturer uptake is minimal( 7 ). Following a recent worldwide survey of food manufacturers, it was concluded that iodized salt use in processed foods may need to be mandated to secure manufacturer uptake( Reference Ohlhorst, Slavin and Bhide 28 ). The initial report produced by Food Standards Australia New Zealand considering the potential introduction of mandatory iodine fortification in New Zealand examined several fortification options( 29 ). Replacing salt in all processed foods with salt iodized at 15 mg/kg was explored, and was estimated to provide an additional 72 μg iodine/d to women of reproductive age, excluding discretionary salt use. This option was rejected, along with universal salt iodization, in favour of the more logistically feasible alternative of replacing salt in yeast-leavened bread, biscuits, crackers and breakfast cereals with salt iodized at 20–45 mg/kg. The proposed mandate was estimated to provide an additional 64 μg iodine/d to New Zealand women of reproductive age, excluding discretionary salt use( 29 ). Following public consultation, this proposal was also rejected. Major hurdles highlighted by the consultation process were the technological difficulty in iodizing large grains of crystallized rock salt (a common ingredient in crackers) evenly; the cost of running separate production lines for non-iodized export products; and the fact that many biscuits are imported( 7 ). Instead, in late 2009, the fortification of all yeast-leavened bread with salt iodized at 20–65 mg/kg was mandated( 7 ). We estimate the implementation of the mandate has resulted in an average additional iodine intake of 37 μg/d among women before pregnancy, slightly over half the achievable intake had salt in all processed foods been iodized at 15 mg/kg.
Interpretation of the current study should be made in recognition of the approximate nature of the iodine intake results, specifically that they are a sum of number of estimated values and are based on recall. Moreover, to assess the true prevalence of inadequacy of dietary iodine intake, rigorous dietary assessment methods, such as the use of weighed food records over multiple days, should be employed and the group distribution of observed intakes should be adjusted for within-person variation( Reference Carriquiry 30 ). In addition, the iodine content of foods in food composition tables should be both complete and accurate( Reference Gibson 31 ). Due to the challenges of dietary intake assessment, most population estimates of iodine intake are derived from the median iodine concentration of spot urine samples; yet due to the day-to-day variability in dietary intakes, use of a single urinary iodine measurement also leads to overestimation of the percentage of the population below the EAR( Reference Zimmermann and Andersson 32 , Reference Taylor, Carriquiry and Bailey 33 ). A further drawback of the use of urinary iodine to estimate dietary iodine intakes is that the contribution from specific iodine sources, such as fortified foods, cannot be determined. Despite these limitations, urinary iodine is regarded as a reliable indicator of dietary iodine intake during pregnancy( Reference Andersson and de Benoist 6 ). Investigations are underway evaluating the sample size and number of repeated urine samples needed to adjust the population distribution of urinary iodine, which will be of considerable value to improving the accuracy of iodine intake assessment in this life-cycle group( Reference Zimmermann and Andersson 32 ).
The findings of the present study are of particular relevance to governments with established maternal iodine supplement recommendations, as well as to those yet to develop policy on such recommendations. Concerns over the potential for a low level of iodine supplement uptake among women of lower socio-economic position have been raised previously( Reference Zimmermann and Delange 23 ) and here we show that these concerns are valid. The ethics of proceeding with such an intervention are thus questionable, as public health strategies should aim to raise the average health of the population without widening disparities( Reference Marmot, Allen and Bell 34 ). While universal salt iodization remains the key strategy to prevent iodine deficiency, in its absence the widespread mandatory fortification of processed foods may be the best and most equitable means of providing adequate iodine to women throughout pregnancy. Encouraging an increase in the use of iodized salt in the home is also at odds with current public health recommendations to reduce Na intakes. A further alternative is to increase the concentration of salt iodization. In Switzerland, where 95 % of households and 70 % of food manufacturers use iodized salt, iodization was increased from 15 to 20 mg/kg salt in 1998( Reference Zimmermann, Aeberli and Torresani 35 ). Both pregnant women and schoolchildren in Switzerland now have adequate iodine intakes( Reference Zimmermann, Aeberli and Torresani 35 ), demonstrating that it is possible to safely meet the needs of most life-cycle groups in a population with a salt iodization strategy.
Acknowledgements
Sources of funding: This study was funded by the University of Otago, Dunedin, New Zealand. Conflict of interest: The authors declare no conflicts of interest. Authors’ contributions: S.R.M. designed the study and the data collection tools; monitored the data collection; cleaned and analysed the data; drafted the paper; and is guarantor. L.A.H. conceived the idea for the study; was involved in study design; monitored data collection; and revised the draft paper. Both authors read and approved the final manuscript. Acknowledgements: The authors thank Andrew Gray (Biostatistician, Department of Preventive and Social Medicine, University of Otago) for his contribution to survey design; dietetic students (Department of Human Nutrition, University of Otago) for participant recruitment and data entry; and Rosalind Gibson (Professor, Department of Human Nutrition, University of Otago) for her advice on the draft manuscript.






