Article contents
Take a deep breath and digest the material: organoids and biomaterials of the respiratory and digestive systems
Published online by Cambridge University Press: 14 August 2017
Abstract
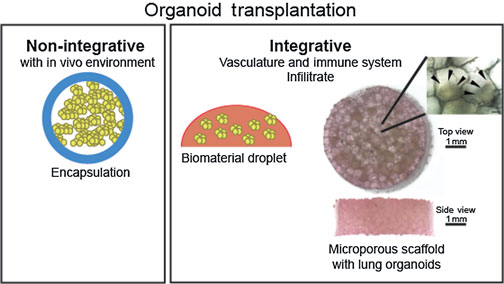
Human organoid models recapitulate many aspects of the complex composition and function of native organs. One of the main challenges in developing these models is the growth and maintenance of three-dimensional tissue structures and proper cellular organization that enable function. Biomaterials play an important role by providing a defined and tunable three-dimensional environment that is required for complex cellular organization and organoid growth in vitro or in vivo. This review summarizes organoids of the respiratory and digestive system, and the use of biomaterials to improve upon these model systems.
- Type
- Biomaterials for 3D Cell Biology Prospective Article
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
- 4
- Cited by


