Article contents
Fiedlerite: revised chemical formula [Pb3Cl4F(OH)·H2O], OD description and crystal structure refinement of the two MDO polytypes
Published online by Cambridge University Press: 05 July 2018
Abstract
The chemical formula of fiedlerite, a rare hydrated lead halide, has been revised. The mineral is now known to contain also fluorine, and the new, correct formula is Pb3Cl4F(OH)·H2O. X-ray diffraction studies on fiedlerite from Laurion, Greece (the type locality), and from Baratti, Italy (the second known occurrence), revealed its Order-Disorder (OD) character. All structures within this OD family can be built up by layers of the same kind. The two polytypes with Maximum Degree of Order (MDO) display triclinic and monoclinic symmetry, with one and two OD layers, respectively, in the unit cell. On these grounds the nomenclature of fiedlerite has been revised, and the mineral is designated together with the polytype suffix (i.e. fiedlerite-1A, fiedlerite-2M). The crystal structures of the two MDO polytypes of fiedlerite have been solved and refined: fiedlerite-1A: P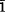 , a = 8.574(3) Å, b = 8.045(4), c = 7.276(2), α = 89.96(4)° β = 102.05(4), γ = 103.45(4), R = 0.092; fiedlerite-2M: P21/a, a = 16.681(4) Å, b = 8.043(3), c = 7.281(2), β = 102.56(4)°, R = 0.061. In both structures Pb is eight-coordinated by different ligands [CI−, F−, (OH)−, H2O] that define bicapped trigonal prisms.
, a = 8.574(3) Å, b = 8.045(4), c = 7.276(2), α = 89.96(4)° β = 102.05(4), γ = 103.45(4), R = 0.092; fiedlerite-2M: P21/a, a = 16.681(4) Å, b = 8.043(3), c = 7.281(2), β = 102.56(4)°, R = 0.061. In both structures Pb is eight-coordinated by different ligands [CI−, F−, (OH)−, H2O] that define bicapped trigonal prisms.
- Type
- Crystal Structure
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 1994
References
- 7
- Cited by




