Editors’ Introduction
Schizophrenia occurs across cultures though actual presentation and help-seeking as well as outcome will depend upon a number of factors. Jablensky in this chapter reminds us that the comparative study of schizophrenia and other psychoses across different populations and cultures gained in scope and momentum with the research programme of the World Health Organization from the 1960s onwards. These studies set the scene and actual standards for cross-cultural epidemiology. He suggests that schizophrenia essentially represents a spectrum of clinical syndromes with some internal cohesion and likely shared genomic underpinnings. The existence of a specific brain disease (or diseases) underlying these conditions points to a schizophrenia syndrome. This remains a hypothesis, notwithstanding the variety of neurobiological and cognitive features or tentative susceptibility genes associated with the disorder. He argues that the question whether cases diagnosed as schizophrenia in different cultures are phenotypically homologous is of critical importance, considering that the biological basis of the disorder still eludes us and no objective diagnostic test is available. He concludes that at present, no single, or major, environmental risk factor influencing the incidence of schizophrenia or other psychoses has been conclusively demonstrated. It is therefore, inevitable that studies using large samples may well give us further clues and direction to evaluate potential risk factors, antecedents and predictors, for which the present evidence is inconclusive.
Introduction
Interest in the manifestations and frequency of mental disorders in non-Western cultures, primarily the psychoses, dates back to the colonial era. In the nineteenth century, British, Dutch and French colonial administrators imported into their overseas dependencies the ‘enlightened’ asylum model of care for the mentally ill and built mental hospitals for custodial care of patients with intractable chronic illness and ‘dangerous’ psychotics. In 1903, Kraepelin travelled to Java and, after several weeks spent at the Buitenzorg (now Bogor) hospital, came to the conclusion that the basic forms of dementia praecox and manic-depressive insanity in the Javanese were generically the same as those in Europe, though ‘racial characteristics, religion and customs’ might modify their clinical manifestations. Although Kraepelin saw in this primarily a confirmation of his nosological system, he anticipated ‘rich rewards’ for the potential of comparative research to ‘throw light on the causes of mental disorder’ and proposed ‘comparative psychiatry’ as the systematic study of mental disorders and personality traits across different cultures (Kraepelin, Reference Kraepelin, Hirsch and Shepherd1904).
However, during the decades to follow, the sources on the epidemiology of schizophrenia and other psychotic disorders in non-European cultures remained restricted to mental hospital statistics or rudimentary field surveys undertaken by psychiatrists or colonial public health administrators. Typically, the conclusion was that ‘true’ schizophrenia and depression were rare among indigenous peoples, but that chronic psychotics were well tolerated in the community. The ideological framework in which such early observations were embedded varied between benign paternalism and overt racism. In a monograph published by the World Health Organization, Carothers (Reference Carothers1953) claimed that the paucity of structured delusional contents and the lack of systematization of delusions in ‘the African’ could be explained by a congenital underdevelopment of the frontal lobes of the brain. Similar assumptions led other authors to conclude that depression was rare in sub-Saharan Africa or in Asia because of the lack of Judaeo-Christian cultural values which made the experience of guilt possible.
The sketchy and often distorted picture of the epidemiology of psychoses in the developing countries started to change in the post-colonial era when locally born psychiatrists, educated in the West, entered practice and teaching in their countries. Though trained in the colonial metropolis, they were keen to understand the nature of mental disorders in their own cultures and to introduce culturally appropriate alternatives to the colonial mental hospital, such as the ‘psychiatric village’ (German, Reference German1972). In a series of studies on schizophrenia among the Yoruba in Nigeria, Lambo (Reference Lambo, De Reuck and Porter1965) pointed to the limitations of the Western diagnostic concepts when applied to African cultures. In Asia, Yap (Reference Yap1974) charted systematically the so-called ‘culture-bound syndromes’ and highlighted their differentiation from schizophrenia. The first epidemiological surveys which generated incidence and prevalence data on psychoses, including schizophrenia, were carried out by Rin and Lin (Reference Rin and Lin1962) in Taiwan; Leighton et al. (Reference Leighton, Lambo, Hughes, Leighton, Murphy and Macklin1963) in Nigeria; and Raman and Murphy (Reference Raman and Murphy1972) in Mauritius. In addition to indigenous investigators, European and North American psychiatrists and anthropologists laid the foundations of cross-cultural psychiatry in which psychosis research featured prominently. Since the late 1950s, a number of epidemiological surveys were carried out in India and China. Although the methods and diagnostic criteria used were rarely described, these surveys provided data of considerable historical interest on general trends and patterns, reviewed by Murphy (Reference Murphy1982).
The comparative study of schizophrenia and other psychoses across different populations and cultures gained in scope and momentum with the research programme of the World Health Organization initiated in the 1960s. Two multi-centre studies, the International Pilot Study of Schizophrenia (IPSS; WHO, 1973, 1979; Leff et al., Reference Leff, Sartorius, Jablensky, Korten and Ernberg1992) and Determinants of Outcome of Severe Mental Disorders (Sartorius et al., Reference Sartorius, Jablensky, Korten, Ernberg, Anker, Cooper and Day1986; Jablensky et al., Reference Jablensky, Sartorius, Ernberg, Anker, Korten, Cooper, Day and Bertelsen1992) generated a wealth of cross-sectional and follow-up data on over 2,000 cases of schizophrenia and related disorders in 16 geographically defined areas in 12 countries in Africa, the Americas, Asia and Europe. These studies utilized for the first time standardized diagnostic criteria and assessment methods in community and hospital-based data collection by teams of local psychiatrists and other mental health workers who not only had been trained to use such research tools but participated in their development. Although the areas covered by the WHO studies were not exhaustive of all the variation that may exist in the incidence of schizophrenia and related conditions, this research provided a unique database enabling direct comparisons of the population rates, psychopathology and outcomes of the major psychoses across various cultures.
Schizophrenia: Phenotypic Comparability across Populations
Despite the availability of ICD-10 and DSM-IV/DSM-5 criteria that aim to facilitate its reliable diagnostic identification, schizophrenia essentially represents a spectrum of clinical syndromes with some internal cohesion and likely shared genomic underpinnings. The existence of a specific brain disease (or diseases) underlying these syndromes is still a hypothesis, notwithstanding the variety of neurobiological and cognitive features or tentative susceptibility genes, associated with the disorders. Thus, the question whether cases diagnosed as schizophrenia in different cultures are phenotypically homologous is of critical importance, considering that the biological basis of the disorder still eludes us and no objective diagnostic test is available. To claim that schizophrenia and its spectrum disorders are universal implies that their features can be reliably identified in different populations; consistent associations with age and gender are present; and course, outcome, and response to treatment show common patterns. Importantly, no population has yet been found to be free of schizophrenia and its related disorders. Although no single symptom is pathognomonic, the overall clinical presentation of schizophrenia is remarkably similar across cultures. Acutely ill patients in different cultural settings describe the same characteristic symptoms, such as hallucinatory voices commenting in third person on their thoughts and actions, thoughts being taken away or broadcast, or their surroundings being imbued with special meaning. Negative symptoms, such as psychomotor poverty, social withdrawal, and amotivation, commonly occur irrespective of the cultural setting. The conclusion that patients diagnosed with schizophrenia in different cultures suffer from the same disorder is further supported by the similar age- and sex-specific distribution of the onset of symptoms, which peak in late adolescence or early adulthood and, in females, have a second, lower peak after age 35. Considering the variety of social norms and beliefs about illness across cultures, the similar ways in which the core symptoms of schizophrenia are experienced and described by people in various cultures is striking, suggesting that the pathophysiological basis of the disorder may be similar in different populations.
Notwithstanding such similarities, variations seem to exist that may affect its recognition and treatment. Lambo (Reference Lambo, De Reuck and Porter1965) described a characteristic symptom-complex in Nigeria consisting of anxiety, depression, vague hypochondriacal concerns, bizarre magico-mystical ideas, episodic twilight or confusional states, atypical depersonalization, emotional liability, and retrospective falsification of memory based on hallucinations or delusions. Variants of the syndrome, such as an acute onset form and a catatonic subtype appear to be more common in traditional rural communities. In the WHO ten-country study (Jablensky et al., Reference Jablensky, Sartorius, Ernberg, Anker, Korten, Cooper, Day and Bertelsen1992) acute onset characterized 40.3% and catatonic schizophrenia 10.3% of all the cases in the low-income countries, compared to 10.9% and 1.2% in the high-income countries respectively. A common clinical problem in some low-income countries is the differentiation of schizophrenia from psychoses due to infectious or parasitic diseases. In particular, African trypanosomiasis often results in a symptomatic psychosis which has a slow, insidious onset and may mimic schizophrenia. Since a variety of infectious, parasitic, and nutritional diseases are endemic in the developing world, it has been suggested that a high proportion of the cases of schizophrenia in those populations may in fact be symptomatic psychoses accompanying physical diseases. However, among some 500 individuals with psychotic illnesses, screened in India and Nigeria for the WHO ten-country study, only 11.7 per cent were excluded on grounds of having a physical disease that might explain their psychotic symptoms. Problems of differential diagnosis may arise in respect of organic brain disorders, but it is unlikely that the majority of schizophrenic illnesses in the Third World can be attributed to underlying organic aetiology.
All this being said, schizophrenic disorders in non-Western populations can be reliably distinguished from the acute transient psychoses, the so-called culture-bound syndromes, and probably the affective disorders, although the boundary with the latter has not been sufficiently explored and some symptomatic overlap may exist. Family morbidity data are still scarce but where such information is available, it suggests that genetic factors contribute to the transmission of schizophrenia in the same way as in the developed countries.
Prevalence
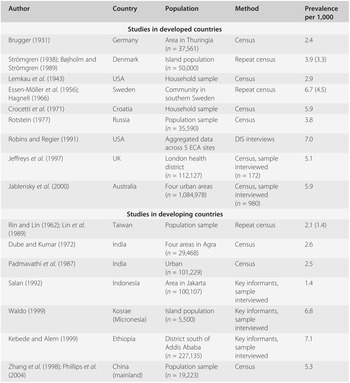
Table 19.1 presents an overview of prevalence studies conducted in different populations at different times, selected on broad criteria of sample representativeness and of diagnostic assessment likely to be compatible with present-day criteria. The studies differ widely in many respects of methodology but have in common a high intensity of case finding. The term ‘census’ refers to surveys aiming at ascertaining every member of an entire community or a population sample. Several were repeat surveys, in which the original sample was traced and re-examined after an interval of ten or more years (the findings on follow-up are quoted in brackets).
Table 19.1 Selected prevalence studies of schizophrenia
| Author | Country | Population | Method | Prevalence per 1,000 |
|---|---|---|---|---|
| Studies in developed countries | ||||
| Brugger (Reference Brugger1931) | Germany | Area in Thuringia (n = 37,561) | Census | 2.4 |
| Strömgren (Reference Strömgren1938); Bøjholm and Strömgren (Reference Bøjholm and Strömgren1989) | Denmark | Island population (n = 50,000) | Repeat census | 3.9 (3.3) |
| Lemkau et al. (Reference Lemkau, Tietze and Cooper1943) | USA | Household sample | Census | 2.9 |
| Essen-Möller et al. (Reference Essen-Möller, Larsson, Uddenberg and White1956); Hagnell (Reference Hagnell1966) | Sweden | Community in southern Sweden | Repeat census | 6.7 (4.5) |
| Crocetti et al. (Reference Crocetti, Lemkau, Kulcar and Kesic1971) | Croatia | Household sample | Census | 5.9 |
| Rotstein (Reference Rotstein1977) | Russia | Population sample (n = 35,590) | Census | 3.8 |
| Robins and Regier (Reference Robins and Regier1991) | USA | Aggregated data across 5 ECA sites | DIS interviews | 7.0 |
| Jeffreys et al. (Reference Jeffreys, Harvey, McNaught, Quayle, King and Bird1997) | UK | London health district (n = 112,127) | Census, sample interviewed (n = 172) | 5.1 |
| Jablensky et al. (Reference Jablensky, McGrath, Herrman, Castle, Gureje, Evans, Carr, Morgan, Korten and Harvey2000) | Australia | Four urban areas (n = 1,084,978) | Census, sample interviewed (n = 980) | 5.9 |
| Studies in developing countries | ||||
|---|---|---|---|---|
| Rin and Lin (Reference Rin and Lin1962); Lin et al. (Reference Lin, Chu and Rin1989) | Taiwan | Population sample | Repeat census | 2.1 (1.4) |
| Dube and Kumar (Reference Dube and Kumar1972) | India | Four areas in Agra (n = 29,468) | Census | 2.6 |
| Padmavathi et al. (Reference Padmavathi, Rajkumar, Kumar and Manoharan1987) | India | Urban (n = 101,229) | Census | 2.5 |
| Salan (Reference Salan1992) | Indonesia | Area in Jakarta (n = 100,107) | Key informants, sample interviewed | 1.4 |
| Waldo (Reference Waldo1999) | Kosrae (Micronesia) | Island population (n = 5,500) | Key informants, sample interviewed | 6.8 |
| Kebede and Alem (Reference Kebede and Alem1999) | Ethiopia | District south of Addis Ababa (n = 227,135) | Key informants, sample interviewed | 7.1 |
| Zhang et al. (Reference Zhang, Shen and Li1998); Phillips et al. (Reference Phillips, Yang, Li and Li2004) | China (mainland) | Population sample (n = 19,223) | Census | 5.3 |
These studies have produced point prevalence estimates in the range of 1.4 to 7.1 per 1,000 population at risk. However, in most instances these are raw (non-standardized) figures which may not be directly comparable due to demographic confounders such as age structure of the population, mortality and migration, and thus may not reflect the true variation across different populations. Thus, the question whether any major differences exist in the prevalence of schizophrenia in different populations and cultures has no simple answer. The majority of studies have found similar prevalence rates, though a small number of populations (referred to later) clearly deviate from the central tendency. The magnitude of such deviations in schizophrenia is typically modest when compared to other multifactorial diseases, such as diabetes, ischaemic heart disease or multiple sclerosis, in which 30-fold (or greater) differences in prevalence across populations are not uncommon.
Incidence
Incidence rates provide a better estimate of the ‘force of morbidity’ (the probability of disease occurrence at a given point in time). The estimation of incidence depends on how reliably the point of onset can be identified. Since it is not possible at present to determine with any accuracy the beginnings of the putative cerebral dysfunction underlying schizophrenia, the onset of the disorder is usually defined as the point in time when its symptoms reach the threshold of recognition. The first hospitalization is not a good index, since the interval between the ‘true’ onset of overt symptoms and the point at which diagnosis is made and treatment initiated (the ‘duration of untreated psychosis’, or DUP) is likely to vary across different settings and cultures. A better approximation is provided by the time of the first contact with any psychiatric or general medical service at which an incipient or ongoing psychotic illness is recognized and diagnosed for the first time.
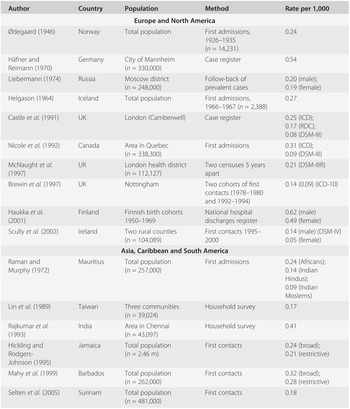
Table 19.2 presents findings from 13 incidence studies of schizophrenia. Studies that have used a ‘broad’ definition of schizophrenia (ICD-8 or ICD-9) suggest that rates based on first admissions or first contacts vary about threefold, between 0.17 and 0.54 per 1,000 population per year. Studies using more restrictive criteria, such as the Research Diagnostic Criteria (Spitzer et al., Reference Spitzer, Endicott and Robins1978), DSM-III and its successors, or ICD-10, report incidence rates that are two to three times lower than those based on ‘broad’ criteria.
| Author | Country | Population | Method | Rate per 1,000 |
|---|---|---|---|---|
| Europe and North America | ||||
| Ødegaard (Reference Ødegaard1946) | Norway | Total population | First admissions, 1926–1935 (n = 14,231) | 0.24 |
| Häfner and Reimann (Reference Häfner, Reimann, Hare and Wing1970) | Germany | City of Mannheim (n = 330,000) | Case register | 0.54 |
| Liebermann (Reference Liebermann1974) | Russia | Moscow district (n = 248,000) | Follow-back of prevalent cases | 0.20 (male);0.19 (female) |
| Helgason (Reference Helgason1964) | Iceland | Total population | First admissions, 1966–1967 (n = 2,388) | 0.27 |
| Castle et al. (Reference Castle, Wessely, Der and Murray1991) | UK | London (Camberwell) | Case register | 0.25 (ICD);0.17 (RDC);0.08 (DSM-III) |
| Nicole et al. (Reference Nicole, Lesage and Lalonde1992) | Canada | Area in Quebec (n = 338,300) | First admissions | 0.31 (ICD);0.09 (DSM-III) |
| McNaught et al. (Reference McNaught, Jeffreys and Harvey1997) | UK | London health district (n = 112,127) | Two censuses 5 years apart | 0.21 (DSM-IIIR) |
| Brewin et al. (Reference Brewin, Cantwell, Dalkin and Fox1997) | UK | Nottingham | Two cohorts of first contacts (1978–1980 and 1992–1994) | 0.14 (0.09) (ICD-10) |
| Haukka et al. (Reference Haukka, Suvisaari, Varilo and Lönnqvist2001) | Finland | Finnish birth cohorts 1950–1969 | National hospital discharges register | 0.62 (male)0.49 (female) |
| Scully et al. (Reference Scully, Quinn and Morgan2002) | Ireland | Two rural counties (n = 104,089) | First contacts 1995–2000 | 0.14 (male) (DSM-IV)0.05 (female) |
| Asia, Caribbean and South America | ||||
|---|---|---|---|---|
| Raman and Murphy (Reference Raman and Murphy1972) | Mauritius | Total population (n = 257,000) | First admissions | 0.24 (Africans);0.14 (Indian Hindus);0.09 (Indian Moslems) |
| Lin et al. (Reference Lin, Chu and Rin1989) | Taiwan | Three communities (n = 39,024) | Household survey | 0.17 |
| Rajkumar et al. (Reference Rajkumar, Padmavathi and Thara1993) | India | Area in Chennai (n = 43,097) | Household survey | 0.41 |
| Hickling and Rodgers-Johnson (Reference Hickling and Rodgers-Johnson1995) | Jamaica | Total population (n = 2.46 m) | First contacts | 0.24 (broad);0.21 (restrictive) |
| Mahy et al. (Reference Mahy, Mallett, Leff and Bhugra1999) | Barbados | Total population (n = 262,000) | First contacts | 0.32 (broad);0.28 (restrictive) |
| Selten et al. (Reference Selten, Zeyl, Dwarkasing, Lumsden, Kahn and van Harten2005) | Surinam | Total population (n = 481,000) | First contacts | 0.18 |
Comparative Incidence Data: The WHO Ten-Country Study
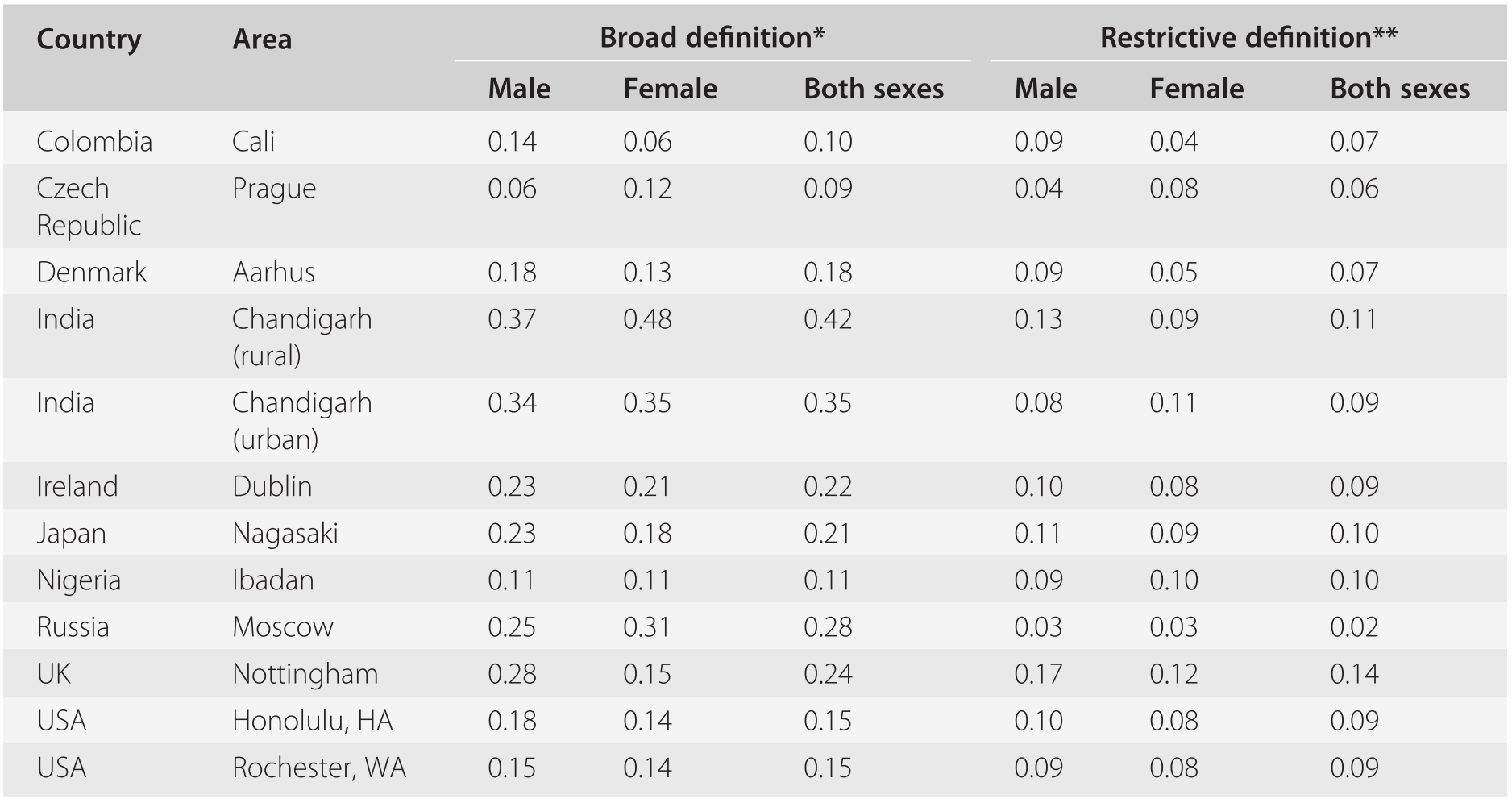
To date, the only study which has generated directly comparable incidence data for different populations is the WHO ten-country investigation (Sartorius et al., Reference Sartorius, Jablensky, Korten, Ernberg, Anker, Cooper and Day1986; Jablensky et al., Reference Jablensky, Sartorius, Ernberg, Anker, Korten, Cooper, Day and Bertelsen1992). Incidence rates were estimated from first-in-lifetime contacts with any ‘helping agency’ (which included traditional healers in the developing countries), monitored prospectively over a 2-year period of case finding. Potential cases and key informants were interviewed by clinicians using standardized instruments, and the timing of onset was ascertained for the majority of patients. For 86 per cent of the 1,022 patients the first manifestation of diagnostic symptoms of schizophrenia was within the year preceding their first contact and, therefore the first-contact rate was accepted as a reasonable proxy for the onset of psychosis. Two definitions of ‘caseness’ were used: a ‘broad’ clinical classification comprising ICD-9 schizophrenia and paranoid psychoses, and a restrictive definition, which included core or ‘nuclear’ schizophrenia with Schneiderian first-rank symptoms (Wing et al., Reference Wing, Cooper and Sartorius1974). The rates for the 12 study areas are shown in Table 19.3.
| Country | Area | Broad definition* | Restrictive definition** | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Both sexes | Male | Female | Both sexes | ||
| Colombia | Cali | 0.14 | 0.06 | 0.10 | 0.09 | 0.04 | 0.07 |
| Czech Republic | Prague | 0.06 | 0.12 | 0.09 | 0.04 | 0.08 | 0.06 |
| Denmark | Aarhus | 0.18 | 0.13 | 0.18 | 0.09 | 0.05 | 0.07 |
| India | Chandigarh (rural) | 0.37 | 0.48 | 0.42 | 0.13 | 0.09 | 0.11 |
| India | Chandigarh (urban) | 0.34 | 0.35 | 0.35 | 0.08 | 0.11 | 0.09 |
| Ireland | Dublin | 0.23 | 0.21 | 0.22 | 0.10 | 0.08 | 0.09 |
| Japan | Nagasaki | 0.23 | 0.18 | 0.21 | 0.11 | 0.09 | 0.10 |
| Nigeria | Ibadan | 0.11 | 0.11 | 0.11 | 0.09 | 0.10 | 0.10 |
| Russia | Moscow | 0.25 | 0.31 | 0.28 | 0.03 | 0.03 | 0.02 |
| UK | Nottingham | 0.28 | 0.15 | 0.24 | 0.17 | 0.12 | 0.14 |
| USA | Honolulu, HA | 0.18 | 0.14 | 0.15 | 0.10 | 0.08 | 0.09 |
| USA | Rochester, WA | 0.15 | 0.14 | 0.15 | 0.09 | 0.08 | 0.09 |
Notes
* ICD-9
** Diagnosis of ‘nuclear’ schizophrenia (S+) assigned by the computer algorithm CATEGO (Wing et al., Reference Wing, Cooper and Sartorius1974) on the basis of symptoms subsequently incorporated into the ICD-10 diagnostic criteria for schizophrenia
The differences between the rates of ‘broad’ schizophrenia (0.16–0.42 per 1,000) across the study areas were statistically significant (p < 0.001, two-tailed test); however, those for nuclear schizophrenia were not. Since nuclear schizophrenia represented a subset of the cases of broad schizophrenia, greater scatter and wider confidence intervals could be expected for the nuclear rates. However, this was not the case, suggesting that nuclear schizophrenia is phenotypically more homogeneous and occurs at a similar frequency in different populations. Subsequently, replications of the design of the WHO ten-country study using the same instruments and procedures have been carried out with very similar results by investigators in India (Rajkumar et al., Reference Rajkumar, Padmavathi and Thara1993), the Caribbean (Hickling and Rodgers-Johnson, Reference Hickling and Rodgers-Johnson1995; Mahy et al., Reference Mahy, Mallett, Leff and Bhugra1999), and the UK (McNaught et al., Reference McNaught, Jeffreys and Harvey1997; Brewin et al., Reference Brewin, Cantwell, Dalkin and Fox1997).
Variation in the Incidence and Prevalence of Schizophrenia across Populations: How Much Similarity and How Much Difference?
Two systematic reviews of the literature (Goldner et al., Reference Goldner, Hsu, Waraich and Somers2002; McGrath et al., Reference McGrath, Saha and Welham2004) highlight the existence of variation in schizophrenia rates across geographical regions. A good deal of this variation may be attributed to methodological differences between the studies, including study design and coverage of case finding (hospital-based, field surveys, case registers, birth cohorts), sample size, diagnostic practices, and methods of data analysis. For example, birth cohort studies and case registers tend to produce higher rates than field surveys and hospital admission studies (Bresnahan et al., Reference Bresnahan, Brown, Schaefer, Begg, Wyatt and Susser2000). Notwithstanding such bias and limitations, real variation is undoubtedly present (as in any human disease) and the interesting research questions concern its extent and sources as clues to a better understanding of aetiology. Since schizophrenia is a low incidence disorder (though its chronicity and associated burden of disability place it high on the public health agenda), variation would be much more visible to the naked eye in the comparison of rates obtained from relatively small geographical areas and communities. In a study of an ethnically and socio-economically homogeneous rural region in Ireland with a total population of 29,542 (Youssef et al., Reference Youssef, Kinsella and Waddington1991; Scully et al., Reference Scully, Owens, Kinsella and Waddington2004) the overall prevalence of 3.9 per 1,000 was well within the ‘modal’ range, but analysis by small district electoral divisions revealed highly significant variation in rates, ranging from 0.0 to 29.4 per 1,000. Similar variation has been reported in the Roscommon study, a genetic epidemiological investigation in another region of Ireland (Kendler et al., Reference Kendler, Karkowski-Shuman and Walsh1996). Such local variation stands in stark contrast to the more uniform rates usually found in studies of large urban areas or at national level and is attributable to a number of factors, including spatial clustering of cases due to shared genetic vulnerability within extended pedigrees; differential mobility and mortality; and differential exposure to risk factors influencing intrauterine growth and early neurodevelopment. Such (and other, still to be discovered) effects may give rise to ‘outlier’ pockets of high or low incidence and prevalence which tend to cancel each other out in larger population agglomerations. Their systematic study, though involving considerable methodological difficulties, has been unduly neglected in favour of the ‘macro’ epidemiology of psychoses.
Populations and Groups with Unusually High and Low Rates: Genetic Isolates
Isolate populations are characterized by origins in a small number of ancestors, a high degree of inbreeding, and a restricted admixture of immigrants, due to geographical or cultural seclusion over multiple generations, sometimes ranging over thousands of years. Such populations may vary considerably in size, but are likely to be less heterogeneous with regard to genetic make-up and environmental exposures, than the panmictic (outbred) populations constituting the world’s majority, in which theoretically all individuals are potentially mating partners. The so-called young isolates comprise up to 20–30 generations, and typically have arisen following drastic population size reductions (bottlenecks) due to wars, famine, religious persecution or other cataclysms. Subsequent population expansion results in a more uniform genetic background, including wider intervals of linkage disequilibrium, a more uniform environment and lifestyle, and significantly higher or lower prevalence of certain diseases, including psychiatric disorders. If coupled with availability of genealogical memory or records, such isolates present unique opportunities for genetic linkage and association studies of Mendelian (monogenic) diseases, and, possibly, complex traits, including schizophrenia, bipolar disorder and other psychiatric syndromes (Varilo and Peltonen, Reference Varilo and Peltonen2004).
A number of isolated populations in different parts of the world – including Finland, Iceland and northern Sweden, the Pima Indians, the Bedouins, the inhabitants of the Central Valley of Costa Rica, several areas in Quebec, as well as religious communities, such as the Old Order Amish, the Hutterites and the Mennonites – have been studied by epidemiologists and geneticists with a view to identifying large pedigrees, informative for complex diseases ranging from asthma and diabetes to schizophrenia and bipolar disorder. Not all of these studies have produced incidence and prevalence rates, but several examples where this has been accomplished highlight the extent of variation in the frequency of psychoses that exists in such unusual groups.
High rates of psychoses (two to three times the national or regional rate) have been reported for population isolates in northern Sweden (Böök et al., Reference Böök, Wetterberg and Modrzewska1978) and several areas in Finland (Hovatta et al., Reference Hovatta, Terwilliger, Lichtermann, Mäkikyrö, Suvisaari, Peltonen and Lönnqvist1997). Though the whole population of Finland shares some features of an ancient isolate (approximately 2,000 years), the northern and eastern regions of Finland have been settled relatively recently (in the sixteenth to seventeenth centuries) and one particular sub-region with a current population of 18,000 was founded by 40 families at the end of the seventeenth century, i.e. 12 generations back (Arjärvi et al., Reference Arajärvi, Haukka and Varilo2004). Genetic-epidemiological studies in this isolate estimate the lifetime risk of schizophrenia at 2.2 per cent, compared to 1.2 per cent for the whole of Finland (Hovatta et al., Reference Hovatta, Terwilliger, Lichtermann, Mäkikyrö, Suvisaari, Peltonen and Lönnqvist1997). A recent case-register based study of a birth cohort (14,817 individuals) from this region established a lifetime prevalence of 1.5 per cent for schizophrenia spectrum psychotic disorders (Arajärvi et al., Reference Arajärvi, Suvisaari and Suokas2005).
Daghestan in the northern Caucasus (Russian Federation) is a region that has been inhabited for over 3,000 years by small ethnic groups constituting together at least five genetically distinct populations, varying considerably in their morbidity patterns. The highest lifetime risk for schizophrenia (4.95%) has been reported from one such highland subisolate of approximately 3,000 members (Bulayeva et al., Reference Bulayeva, Leal and Pavlova2005). The population of the whole region consists of 26 ethnic subisolates in which the lifetime risk of schizophrenia was found to vary from 1.46 per cent to 4.95 per cent, i.e. the highest risk estimate ever reported for an isolate population.
The population of the Palau islands (Micronesia), currently 20,470 people, has been geographically and ethnically isolated from other Pacific populations for nearly 2,000 years. A genetic epidemiological study of treated cases estimated the lifetime risk of schizophrenia at 2.77% in males and 1.99% in females, i.e. high in excess of the ‘modal’ risk of about 1% reported for large outbred populations. All of the 160 Palau cases were concentrated in 59 families, each traceable to a single common founder, with 11 of them having 5 to 11 affected members each (Myles-Worsley et al., Reference Myles-Worsley, Coon and Tiobech1999).
At the other extreme, the lowest known prevalence rate of schizophrenia in any population (and a very low rate of bipolar disorder) has been found among the Hutterites in South Dakota, a Protestant sect of European descent whose members have lived since the 1870s in closely knit, endogamous rural communities in Manitoba (Canada) and South Dakota (US). According to preserved pedigree records, all of the present 35,000 Hutterites are descendants of fewer than 90 ancestors who lived in the eighteenth and early nineteenth century. Reduced genetic heterogeneity and communal lifestyle with minimum variation in environmental exposures make this population an ideal laboratory for a variety of disease studies (Ober et al., Reference Ober, Abney and McPeek2001; Newman et al., Reference Newman, Hoffjan and Bourgain2004), including psychiatric disorders. An early epidemiological study, in which the entire population of several Hutterite communities was screened, resulted in a schizophrenia lifetime prevalence of 1.1 per 1,000 (Eaton and Weil, Reference Eaton and Weil1955). Subsequent reanalysis of the data using DSM-IIIR criteria (Torrey, Reference Torrey1995), and a repeat survey (Nimgaonkar et al., Reference Nimgaonkar, Fujiwara and Dutta2000) replicated the original finding. Both genetic (low frequency of psychosis-predisposing alleles) and lifestyle factors (protective community support) have been proposed as an explanation for the unusually low rate of psychosis. Negative selection for individuals with schizoid traits who fail to adjust to the communal lifestyle and eventually migrate without leaving progeny has also been suggested, but not definitively proven. Low rates have also been reported for certain Pacific island populations. Two surveys in Taiwan (Rin and Lin, Reference Rin and Lin1962; Lin et al., Reference Lin, Chu and Rin1989), separated by 15 years during which major social changes took place, found that the prevalence of schizophrenia decreased from 2.1 to 1.4 per 1,000. In both surveys, the aboriginal Taiwanese had significantly lower rates than the mainland Chinese who had migrated to the island after World War II.
High Rates of Psychosis in Ethnic Minorities, Migrants and Refugees
The effects of migration on the incidence of psychosis have been studied extensively since the 1930s (Ødegaard, Reference Ødegaard1932). The publication of the first report of an increased prevalence of psychoses among African Caribbean minority groups in the UK (Hemsi, Reference Hemsi1967), was followed by an increasing number of studies showing very high incidence rates of schizophrenia (about 0.6 per 1000) in the African Caribbean population in the UK (Bhugra et al., Reference Bhugra, Leff, Mallett, Der, Corridan and Rudge1997; Harrison et al., Reference Harrison, Glazebrook, Brewin, Cantwell, Dalkin, Fox, Jones and Medley1997). A recent systematic review of the incidence of psychotic disorders in Caribbean-born migrants and their descendants in the UK (Tortelli et al., Reference Tortelli, Errazuriz and Croudace2015) established that higher incidence rates among black Caribbean groups have been in existence for more than 60 years (incidence rate ratio, IRR = 4.7 relative to the reference ‘white British’ population). Moreover, the authors found that ethnic minorities, including the black Caribbeans, having been exposed to cumulative social disadvantage since childhood, had ‘more complex pathways to care … and may receive worse mental health care’. Similar conclusions were drawn from a large school-based study of ethnic minority youth in the Netherlands (Adriaanse et al., Reference Adriaanse, van Domburgh, Hoek, Susser, Doreleijers and Veling2015), comparing the prevalence of psychotic experiences in Moroccan-Dutch (9.5%) and Turkish-Dutch (7.1%) minority children and adolescents relative to their native Dutch counterparts (3.1%). Perceived discrimination was found to be the main psychosocial factor associated with the findings. Such elevated rates of psychotic experiences, particularly in the Moroccan-Dutch children seemed to parallel the very high incidence rates of adult psychotic disorders in this minority ethnic group and might be predictive of adult psychopathology. Interestingly, minority status is not necessarily associated with an elevated risk for psychotic disorders, as shown by Suvisaari et al. (Reference Suvisaari, Opler, Lindbohm and Sallmen2014) in a study comparing the Swedish-speaking minority and the Finnish-speaking majority in Finland. While Swedish speakers made up approximately 5% of the population, their prevalence of schizophrenia spectrum disorders amounted to 0.7%, compared to 1.5% in the Finnish-speaking majority. However, as a group Swedish speakers tended to have a higher socio-economic position and longer life expectancy, leading the authors to conclude that ‘social capital may be protective against schizophrenia’.
Refugees and asylum seekers represent a population group which is ethnically heterogeneous but nearly homogeneous in their exposure to severe stress related to war, natural disasters and political, racial or religious persecution. According to United Nations data on forced displacement and migration, in 2015 some 244 million people (3.3 per cent of the world’s population) have been living outside their country of origin (Katona, Reference Katona2016), and their number is rapidly increasing. With some exceptions (e.g. Logue et al., Reference Logue, Amstadter and Baker2015) psychiatric research on the whole is not catching up with these massive population shifts. However, one recent well-designed epidemiological study (Hollander et al., Reference Hollander, Dal, Lewis, Magnusson, Kirkbride and Dalman2016) has addressed the issue of refugee migration and risk of schizophrenia in a large cohort study of 1.3 million people in Sweden. The cohort was comprised of 88.4% people born in Sweden of two Swedish parents; 9.8% non-refugee migrants from the Middle East, north and sub-Saharan Africa, Asia and eastern Europe; and 1.8% refugees from the same regions. The linked data were extracted from the Swedish national registers which include all people resident in Sweden since 1932 who have been assigned a personal identity number, anonymized for the purposes of research. The database amounted to 8.9 million person years of follow-up. The incidence rate of non-affective psychotic disorders was 38.5 per 100,000 person years in the Swedish-born; 80.4 per 100,000 in the migrants; and 126.4 per 100,000 in the refugees. This corresponded to hazard ratios of 1.75 in migrants and 2.90 in refugees, compared to the Swedish born. Thus, refugees were 1.66 times more likely than migrants to develop non-affective psychotic disorders. The authors conclude that refugees face ‘substantially elevated rates of schizophrenia and other non-affective psychoses, in addition to … other mental, physical and social inequalities … our findings support the possibility that exposure to psychosocial adversity increases the risk of psychosis’.
Similar findings of nearly fourfold excess over the general population rate have been reported for the Dutch Antillean and Surinamese immigrants (Selten et al., Reference Selten, Slaets and Kahn1997), and for Moroccans and other non-Western immigrants in the Netherlands (Veling et al., Reference Veling, Selten and Veen2006). Research to date has not identified definitively any specific cause explaining this phenomenon. Little evidence has been presented to support earlier suggestions that these psychotic illnesses might be explained as substance-induced episodes or acute transient psychoses. Neither the cross-sectional symptom picture, nor the course and outcome of these disorders present any atypical features that would set them apart from ICD-10 or DSM-IIIR/DSM-IV schizophrenia (Harrison et al., Reference Harrison, Amin, Singh, Croudace and Jones1999; Hutchinson et al., Reference Hutchinson, Takei, Sham, Harvey and Murray1999). Incidence studies in the Caribbean (Hickling and Rodgers-Johnson, Reference Hickling and Rodgers-Johnson1995; Bhugra et al., Reference Bhugra, Mallett and Leff1999; Selten et al., Reference Selten, Zeyl, Dwarkasing, Lumsden, Kahn and van Harten2005) do not indicate any excess schizophrenia morbidity in the countries of origin from which migrants are recruited. Explanations in terms of biological risk factors, such as increased incidence of obstetric complications or maternal influenza, have been put to the test but found no support (Hutchinson et al., Reference Hutchinson, Takei and Bhugra1997; Selten et al., Reference Selten, Slaets and Kahn1998).
A potentially important finding is the increased incidence of schizophrenia among the siblings of second-generation African Caribbean index cases, as compared to the incidence of schizophrenia in the siblings of white index cases with schizophrenia (Hutchinson et al., Reference Hutchinson, Takei and Bhugra1996). Such increases in the morbid risk within sibships (in the absence of a similar increase in the risk among parents) suggest a lowered threshold for the expression of the disorder in carriers of susceptibility alleles that might be induced by environmental stress. Hypotheses involving psychosocial risk factors, such as lack of a supportive community structure, acculturation stress, demoralization resulting from racial discrimination, and blocked opportunity for upward social mobility have been proposed (Bhugra et al., Reference Bhugra, Mallett and Leff1999) but not yet definitively tested. Although psychosocial adversity is most likely to affect the majority of immigrants, a plausible pathogenetic mechanism involving specific gene–environment interactions and linking such stress to the incidence of psychosis remains to be demonstrated.
Course and Outcome
Systematic investigations into the course and outcome of schizophrenia were initiated by Kraepelin (Reference Kraepelin1919) who believed that the natural history of the disorder could provide a provisional validation of the disease concept until final verification could be achieved by establishing the brain pathology and aetiology. Arguably, the greatest extent of variation in schizophrenia across populations and cultures is manifest in the course and outcome of the disorder. Early reports, based on small clinical samples, pointed to a less disabling course and a high rate of recovery from schizophrenic psychoses in developing countries such as Mauritius (Raman and Murphy, Reference Raman and Murphy1972) and Sri Lanka (Waxler, Reference Waxler1979) in cases that, according to ‘Western’ prognostic criteria, should have a poor outcome. Selection bias could not be ruled out in such studies based on hospital admissions; standard assessment procedures and explicit diagnostic criteria were not used; and clinical improvement could have been confounded with the social adjustment many patients achieve in a comparatively undemanding environment. Thus, room was left for doubts about the validity of findings of a better prognosis of schizophrenia in non-Western environments.
Many of these methodological issues were addressed in the WHO multi-centre studies by employing standardized assessment and more refined measures of course and outcome than in previous research. In the International Pilot Study of Schizophrenia (IPSS; WHO 1973, 1979), the 2- and 5-year follow-up assessments of patients indicated significantly higher proportions of patients in India, Colombia and Nigeria having better outcomes on all dimensions than patients in the high-income countries. The initial psychotic episode had remitted during the 5-year follow-up in as many as 42 per cent of the patients in India and 33 per cent of the patients in Nigeria, whereas the majority of patients in the developed high-income countries had experienced persisting psychotic symptoms and disablement. In either setting, patients with good and poor outcomes could not be clearly distinguished on the basis of their initial symptoms, though they all met the ICD-9 criteria for a diagnosis of schizophrenia.
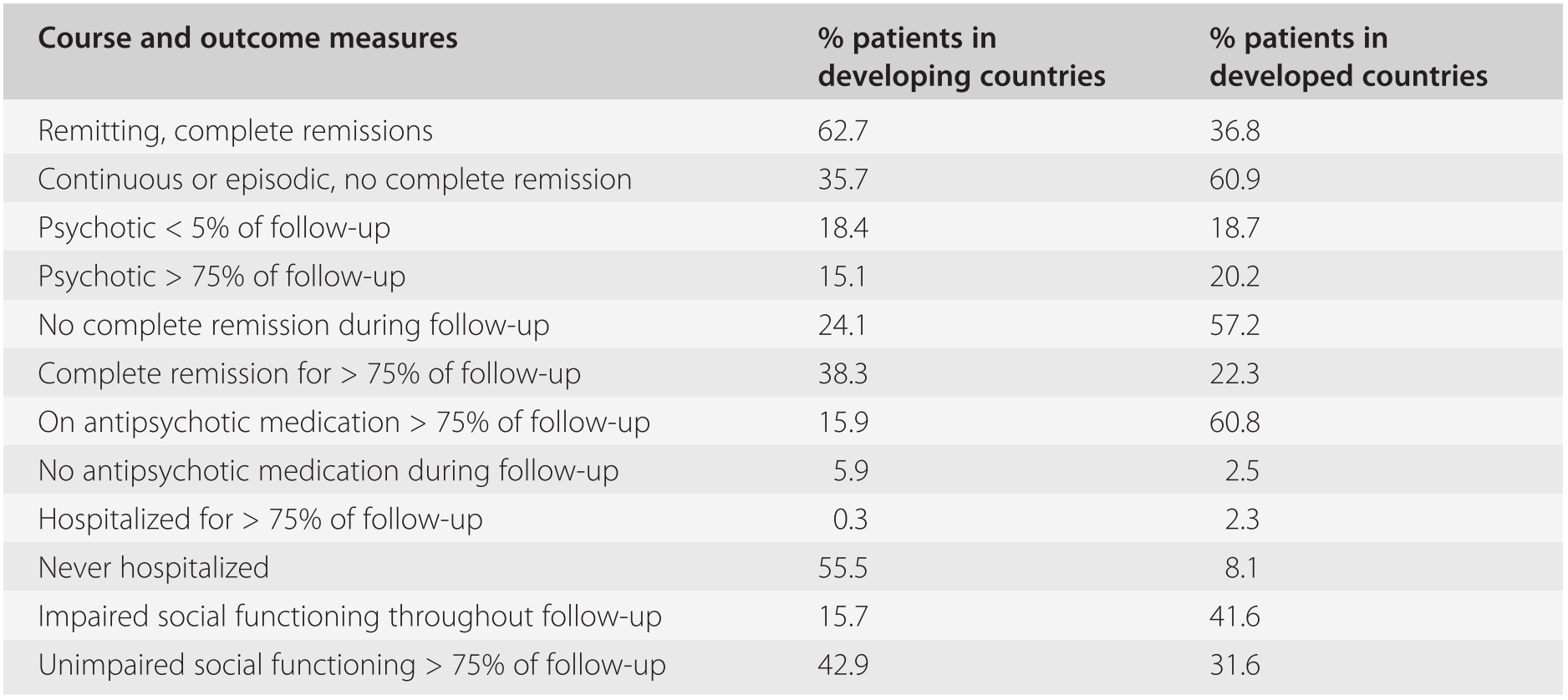
Nevertheless, the IPSS was not free of bias, since patients were recruited from hospitals. Bed availability and admission policies could have led to over-inclusion of chronic cases in the developed countries and of recent-onset, acute cases in the developing countries. Such confounding factors were largely eliminated in the subsequent WHO ten-country study (Jablensky et al., Reference Jablensky, Sartorius, Ernberg, Anker, Korten, Cooper, Day and Bertelsen1992), in which uniformly assessed first-episode cases were assessed upon their first contact with community or hospital services. The 2-year follow-up (and longer-term follow-up in several of the centres) provided ample confirmation of the finding that the outcome of schizophrenia was generally better in developing than in developed countries (Table 19.4).
Table 19.4 Two-year course and outcome in the WHO ten-country study: developed and developing countries
| Course and outcome measures | % patients in developing countries | % patients in developed countries |
|---|---|---|
| Remitting, complete remissions | 62.7 | 36.8 |
| Continuous or episodic, no complete remission | 35.7 | 60.9 |
| Psychotic < 5% of follow-up | 18.4 | 18.7 |
| Psychotic > 75% of follow-up | 15.1 | 20.2 |
| No complete remission during follow-up | 24.1 | 57.2 |
| Complete remission for > 75% of follow-up | 38.3 | 22.3 |
| On antipsychotic medication > 75% of follow-up | 15.9 | 60.8 |
| No antipsychotic medication during follow-up | 5.9 | 2.5 |
| Hospitalized for > 75% of follow-up | 0.3 | 2.3 |
| Never hospitalized | 55.5 | 8.1 |
| Impaired social functioning throughout follow-up | 15.7 | 41.6 |
| Unimpaired social functioning > 75% of follow-up | 42.9 | 31.6 |
Analysis of the data led to the conclusion that the better overall pattern of course and less disabling outcome in the developing countries was primarily due to a significantly greater percentage of patients remaining in a stable remission of symptoms over longer periods after recovery from an acute psychotic episode, rather than to milder or shorter psychotic episodes. This pattern was significantly predicted by setting (developing/developed country), acute onset, being married or cohabiting with a partner, and having access to a supportive social network. Being female was generally associated with a more favourable outcome. The length of remissions was unrelated to antipsychotic treatment, which generally was administered for much shorter periods of time to patients in the low-income countries. Independently of the WHO studies, a high proportion of better outcomes of schizophrenia in developing countries has been reported by numerous investigators (Kulhara and Chandiramani, Reference Kulhara and Chandiramani1988; Ohaeri, Reference Oheari1993; Thara et al., Reference Thara2004).
The factors underlying the better outcome of schizophrenia in developing countries remain insufficiently understood but, in a very general sense, are likely to involve interactions between genetic variation and specific aspects of the environment. Differences in the course and outcome of a disease across and within populations may be related to varying frequencies of predisposing or protective alleles coding for proteins involved in neurodevelopment, neurotransmitter and receptor regulation, or intracerebral signalling between brain subsystems. In a large study conducted by the International Schizophrenia Consortium (de Candia et al., Reference De Candia, Lee, Yang and Gejman2013) additive genetic variation in schizophrenia risk was compared in samples of African descent (N = 2,142) and European descent (N = 4,990). The genetic correlation between the two samples was 0.66 (highly significant), suggesting that ‘many schizophrenia risk alleles are shared across ethnic groups and pre-date the African-European divergence’. While such genetic similarities undoubtedly exist, nothing specific can at present be said as to their role in the course and outcome of schizophrenia. However, a strong effect of the psychosocial environment is entirely plausible, considering the contrasts between developing and developed countries with regard to social support systems, kinship networks and beliefs about mental disease (Warner, Reference Warner1983). It is, therefore, unlikely that the differences in the course and outcome of schizophrenia across populations and cultures could be explained by the operation of a single factor. The observed differences may result from the additive or interactive effects of: (a) genetic and pathophysiological differences between acute and insidiously arising schizophrenic syndromes which may have differential propensities towards recovery and stabilization; (b) lower incidence in traditional societies of the type of chronic stress to which people with schizophrenia are particularly vulnerable; (c) higher probability in traditional societies of an individual–environment fit that minimizes social isolation and withdrawal and prevents the development of secondary disabilities.
As regards (b), the WHO ten-country study found that the index of expressed emotion (EE), a short-range predictor of psychotic relapse, was as effective in Indian families (Wig et al., Reference Wig, Menon and Bedi1987) as in European and North American families, but that high-EE families were significantly rarer in India than in Denmark or the UK. This established a potentially important and specific cultural difference. If this finding could be replicated in other settings in developing countries, the relative rarity of at least one type of pathogenic stress in the daily environment of schizophrenic individuals would be demonstrated. However, it is unlikely that EE is the only type of stress to which schizophrenic individuals respond with psychotic exacerbation. Murphy (Reference Murphy1982) proposed four criteria for schizophrenia-evoking stress: (1) a situation demanding action or decision; (2) complexity or ambiguity of the information supplied to deal with the task; (3) unless resolved, the situation demanding action or decision persists; (4) the person has no ‘escape route’ available. Each one of the components of the putative model may occur at different frequencies in traditional and industrialized societies, a proposition that should be testable epidemiologically or experimentally. As regards (c), the most important differences between traditional cultures and the industrial Western societies concern the sick role and beliefs and practices related to mental illness. The suggestive power of magical-mystic explanations of mental illness and of traditional healing practices may not cure schizophrenia but is likely to lower the barriers to spontaneous recovery and reintegration in the community. Generally, the findings of a better outcome for schizophrenia in traditional societies are compelling and set a research agenda that may lead to discoveries with fundamental implications for the management and treatment of schizophrenia in both developing and developed countries.
Acute and Transient Psychotic Disorders
Acute psychoses, different from schizophrenia or manic-depressive illness, were first described in French psychiatry as bouffées délirantes (Magnan and Legrain, Reference Magnan and Legrain1895), and as cycloid psychoses (Kleist, Reference Kleist1921; Leonhard, Reference Leonhard1995) in German psychiatry. The clinical picture overlaps with the psychogenic psychoses described by Danish psychiatrists (Wimmer, Reference Wimmer1916; Strömgren, Reference Strömgren1986) and the schizophreniform psychoses described by Langfeldt (Reference Langfeldt1939) in Norway. These disorders represent a modest fraction of psychiatric morbidity in Western countries but are considered common in many parts of the developing world. Their correct and timely recognition is important because of their benign prognosis which is quite different from the outcome of schizophrenia or major mood disorders. ICD-10 includes a separate rubric (F23) with five subdivisions and diagnostic guidelines which aim at differentiating such acute psychoses from schizophrenia. Since little is known about their pathophysiology and genetics, this group of disorders provides a rewarding field of inquiry for clinical and epidemiological research. Common features of these states include rapid onset (‘out of the blue’), few prodromal signs, dramatic and variable symptom presentation, short duration and equally rapid recovery with few residual signs. Often, but by no means always, they arise in response to psychosocial or physiological stress, but there is no characteristic family history, and the premorbid personality is inconspicuous. Recurrence of such episodes is the rule but the relapse rate is lower than in schizophrenia or affective disorder.
The French concept of bouffées délirantes is probably the earliest description of an acute transient psychosis. The term refers to a brief non-organic psychosis which typically presents with a sudden onset of fully formed, thematically variable delusions and hallucinations against a background of mild clouding of consciousness and fluctuating affect, and typically results in spontaneous recovery with some probability of relapse. Mental trauma is either absent or plays a minor role in the causation of bouffées délirantes, whose aetiology was primarily attributed to a vulnerable mental constitution. The description of the cycloid psychoses includes sudden onset, pervasive delusional mood, variable delusions, hallucinations in any modality, labile affect and psychomotor disturbances (excitement or inhibition). Stressful life events may precipitate a psychotic episode but the content of the psychotic experiences does not reflect the traumatic event. Leonhard emphasized the polarity of the dominant disturbance in cycloid psychoses and distinguished three subtypes: (1) ‘anxiety-happiness psychosis’ (extreme shifts of affect between intense fear and ecstatic elation); (2) ‘motility psychosis’ (impulsive hypermotility and psychomotor inhibition); (3) ‘confusion psychosis’ (incoherent pressure of speech and mutism). The duration varies from days to a few weeks but recovery is always complete, though there is a risk of further episodes in which much the same symptoms tend to recur.
The concept of psychogenic psychosis, introduced by Sommer (Reference Sommer1894) and later elaborated by Jaspers (Reference Jaspers1963) and Scandinavian psychiatrists, defined a psychotic reaction, originating in traumatic experiences, which is psychologically understandable in terms of several criteria: (1) its content reflects the nature and significance of the psychic trauma; (2) there is a temporal relationship between the trauma and the onset; (3) removal of the traumatizing factor results in recovery; (4) the overall prognosis is good. However, the extent to which transient psychotic illnesses actually meet the criteria set by Jaspers and Wimmer is uncertain as few studies have attempted to explore its validity.
Conclusion: Prospects for Epidemiology in the Search for the Causes of Psychoses
Important insights into the nature and causes of psychotic disorders, primarily schizophrenia and its spectrum, have been gained from population-based studies, although essential questions still remain unanswered. With regard to schizophrenia, the clinical syndrome appears to be robust and identifiable reliably in diverse populations and cultures, suggesting that a common pathophysiology and likely common genetic predisposition underlie its manifestations. At the level of large population aggregates, no major differences in incidence and morbid risk have to date been detected, though small geographical area variations exist and appear to be related to a mix of risk factors whose effects may be attenuated in large, heterogeneous populations.
The study of ‘atypical’ populations, such as genetic isolates or minority groups, may be capable of detecting unusual variations in the incidence of schizophrenia and other psychoses that could provide novel clues to the aetiology and pathogenesis of these disorders. Notwithstanding the difficulties in the genetic dissection of complex disorders, emerging powerful methods of genomic analysis will eventually identify polymorphisms and haplotypes associated with schizophrenia risk. The majority are likely to be in genes of small effect, although one cannot rule out the possibility that rare polymorphisms of moderate or major effect will also be found, especially in isolate populations or at the level of neurocognitive and neurophysiological abnormalities underlying the disorder. Establishing their population frequency and associations with a variety of phenotypes, including personality traits, will be a major task for comparative epidemiology.
At present, no single, or major, environmental risk factor influencing the incidence of schizophrenia or other psychoses has been conclusively demonstrated. Further studies using large samples are required to evaluate potential risk factors, antecedents and predictors, for which the present evidence is inconclusive. The relationship between genotype and phenotype in schizophrenia is likely to be mediated by complex causal pathways involving gene–gene and gene–environment interactions, ‘programmable’ neural substrate, and stochastic events. Three models of the joint effects of genotype and environment have been proposed (Kendler and Eaves, Reference Kendler and Eaves1986): (1) the effects of predisposing genes and environmental factors are additive and increase the risk of disease in a linear fashion; (2) genes control the sensitivity of the brain to environmental insults; and (3) genes influence the likelihood of an individual’s exposure to environmental pathogens, e.g. by fostering certain personality traits.
A complementary research strategy proceeds from evidence that the ICD-10 or DSM-IV clinical diagnoses of schizophrenia and other non-affective psychoses may not represent relevant phenotypes for genetic research (Jablensky, Reference Jablensky2006). This leads to an exploration of alternative, intermediate phenotypes (or ‘endophenotypes’), such as neurocognitive abnormalities, or temperament and character traits associated with schizophrenia that may be expressed in both affected individuals and their asymptomatic biological relatives. A prerequisite for the application of this approach is the establishment of population prevalences for such endophenotypes in epidemiological samples.
Current epidemiological research is increasingly making use of existing large databases, such as cumulative case registers or birth cohorts to test hypotheses about risk factors in case-control designs. Methods of genetic epidemiology are increasingly being integrated within population-based studies. These trends predict a bright future for epidemiology in the unravelling of gene–environment interactions that are likely to be the key to the understanding of the aetiology of psychoses. In this context, research into psychotic disorders in non-Western populations can provide valuable information on the genetic heterogeneity, the impact of the environment, and the course and outcome of psychotic disorders. Both traditional communities and societies undergoing transition in their social organization can contribute critically to the better understanding of the relationships between culture and mental disorder and the variety of human experience in coping with mental illness.