Aldermanite from Tom's quarry in the Kapunda–Angaston area of the Mount Lofty Ranges, South Australia has been characterised by electron microprobe analyses and single-crystal structure analysis. The empirical formula is Na0.72K0.13Ca0.06Mg1.15Al2.92(PO4)2.05[(OH)2.92F2.96]Σ5.88⋅8.91H2O, based on 23 anions. Analysis of a specimen from the type locality, the nearby Klemm's quarry, Moculta, gave a similar formula, Na0.59K0.06Ca0.36Mg0.92Al3.16(PO4)1.97[(OH)4.08F2.70]Σ6.78⋅8.36H2O. Na and F were not analysed in the original description of the mineral. The ideal end-member formula is [Mg(H2O)6][Na(H2O)2Al3(PO4)2(OH)6]⋅H2O, compared to the original formula of Mg5Al12(PO4)8(OH)22⋅nH2O with n ≈ 32. Aldermanite is monoclinic, P21/c with a = 13.524(3), b = 9.958(2), c = 7.013(1) Å and β = 97.40(3)°. The crystal structure of aldermanite is built from sawtooth layers of cis- and trans-corner-connected, Al-centred octahedra, decorated with corner-connected PO4 tetrahedra to give (100) layers of composition Al3(PO4)2(OH,F)6. Interlayer Mg(H2O)6 octahedra and H2O molecules hold the layers together through H bonding. The corner-connected octahedra form 6-membered rings that are centred by 8-coordinated Na and have a topology identical to a 3-octahedra-wide {110} slice of the pyrochlore structure. This pyrochlore element contains intersecting kagomé nets of Al atoms, parallel to (111) and (11$\bar{1}$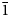 ) of cubic pyrochlore. Minerals of the walentaite group, as well as zirconolite-3O polytypes have the same type of intersecting kagomé nets of small cations.
) of cubic pyrochlore. Minerals of the walentaite group, as well as zirconolite-3O polytypes have the same type of intersecting kagomé nets of small cations.