Melting experiments with plagioclases were performed in the systems Ab-An, Ab-An-H2O, Qz-Ab-An-H2O, and Qz-Or-Ab-An-H2O-CO2. The experimental products were analysed by electron microprobe, and the kinetics of the reactions were studied qualitatively.
Melting of the plagioclase in the system Ab-An (P = 1 atm, T = 1420°C) is very fast in the first minutes but becomes slower with increasing run duration and is incomplete even after 1000 hours. The Ab/An fractionation between melt and residual plagioclase is similar to that described by Bowen (1913).
Melting kinetics of plagioclase in the system Ab-An-H2O (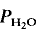 = 5 kbar, T = 1000°C) is controlled by the diffusion of water into the plagioclase structure. Melting is especially fast parallel to the a-axis. The experimental products show separation of melts and crystals.
= 5 kbar, T = 1000°C) is controlled by the diffusion of water into the plagioclase structure. Melting is especially fast parallel to the a-axis. The experimental products show separation of melts and crystals.
In the tonalite system Qz-Ab-An-H2O, equilibrium melting could be observed down to 830°C (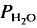 = 2 kbar) but not a lower temperatures. The kinetics of the reaction is enhanced by deficiency or excess of alumina in the aluminosilicate melt surrounding the plagioclase crystals. The fractionation of Ab and An between melt and plagioclase crystals is more pronounced in the presence of quartz than in the Ab-An-H2O system. The ratio An/An + Ab is approximately 0·35 in the melt and 0·85 in the coexisting plagioclase T = 880°C).
= 2 kbar) but not a lower temperatures. The kinetics of the reaction is enhanced by deficiency or excess of alumina in the aluminosilicate melt surrounding the plagioclase crystals. The fractionation of Ab and An between melt and plagioclase crystals is more pronounced in the presence of quartz than in the Ab-An-H2O system. The ratio An/An + Ab is approximately 0·35 in the melt and 0·85 in the coexisting plagioclase T = 880°C).
In the haplogranodiorite system Qz-Or-Ab-An-H2O–CO2, melting reactions were performed at P = 0·5 kbar, T = 880°C, and 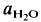 of approximately 0·5. It is assumed that near equilibrium compositions of melt and coexisting residual plagioclase could be obtained in long duration runs (run time = 60 days). The distribution of Ab and An between melt and minerals is similar to that observed in the tonalite system. The partial melt coexisting with an An-rich plagioclase and Or-rich K-feldspar is relatively poor in An.
of approximately 0·5. It is assumed that near equilibrium compositions of melt and coexisting residual plagioclase could be obtained in long duration runs (run time = 60 days). The distribution of Ab and An between melt and minerals is similar to that observed in the tonalite system. The partial melt coexisting with an An-rich plagioclase and Or-rich K-feldspar is relatively poor in An.

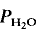 = 5 kbar,
= 5 kbar, 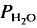 = 2 kbar) but not a lower temperatures. The kinetics of the reaction is enhanced by deficiency or excess of alumina in the aluminosilicate melt surrounding the plagioclase crystals. The fractionation of Ab and An between melt and plagioclase crystals is more pronounced in the presence of quartz than in the Ab-An-H
= 2 kbar) but not a lower temperatures. The kinetics of the reaction is enhanced by deficiency or excess of alumina in the aluminosilicate melt surrounding the plagioclase crystals. The fractionation of Ab and An between melt and plagioclase crystals is more pronounced in the presence of quartz than in the Ab-An-H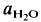 of approximately 0·5. It is assumed that near equilibrium compositions of melt and coexisting residual plagioclase could be obtained in long duration runs (run time = 60 days). The distribution of Ab and An between melt and minerals is similar to that observed in the tonalite system. The partial melt coexisting with an An-rich plagioclase and Or-rich K-feldspar is relatively poor in An.
of approximately 0·5. It is assumed that near equilibrium compositions of melt and coexisting residual plagioclase could be obtained in long duration runs (run time = 60 days). The distribution of Ab and An between melt and minerals is similar to that observed in the tonalite system. The partial melt coexisting with an An-rich plagioclase and Or-rich K-feldspar is relatively poor in An.