The crystal structure of rabejacite from Jáchymov, ideally Ca2[(UO2)4O4(SO4)2](H2O)8, was solved by charge flipping from single-crystal data and refined to R1 = 11.94% for 1422 unique observed reflections [I > 3σ(I)]. According to single-crystal X-ray data, rabejacite is triclinic, space group P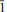 , with a = 8.7434(11), b = 8.309(3), c = 8.8693(10) Å , a = 77.86(2), b = 104.635(11), g = 82.935(18)°, V = 598.8(3) A˚ 3 and Z = 1, with Dcalc = 4.325 g cm–3. The structure refinement proved that rabejacite is related to the zippeite group of minerals, as it is based upon the structural sheets of the zippeite topology of composition [(UO2)4O4(SO4)2]4–. Located in the interlayer between the sheets, which are stacked perpendicular to [010], are Ca2+ cations and H2O groups. Ca2+ ions are [7]-coordinated, by three uranyl O atoms from adjacent sheets and four H2O groups. An additional H2O group, which is not bonded directly to any cation, is located in the interlayer. Along with rabejacite, its Cu-rich variety was found in the specimens examined and characterized structurally. Its crystal structure (R1 = 10.15% for 1049 reflections with I > 3s(I)) is practically the same as that of rabejacite, but there is an additional Cu2+ site located in between pairs of Ca polyhedra. The structural formula is (Ca1.56Cu0.40)Σ1.90[(UO2)4O4(SO4)2](H2O)8, Z = 1. Its existence suggests a greater diversity in zippeite crystal chemistry than was thought previously and also the possibility of a new Cu2+-dominant zippeite mineral besides pseudojohannite.
, with a = 8.7434(11), b = 8.309(3), c = 8.8693(10) Å , a = 77.86(2), b = 104.635(11), g = 82.935(18)°, V = 598.8(3) A˚ 3 and Z = 1, with Dcalc = 4.325 g cm–3. The structure refinement proved that rabejacite is related to the zippeite group of minerals, as it is based upon the structural sheets of the zippeite topology of composition [(UO2)4O4(SO4)2]4–. Located in the interlayer between the sheets, which are stacked perpendicular to [010], are Ca2+ cations and H2O groups. Ca2+ ions are [7]-coordinated, by three uranyl O atoms from adjacent sheets and four H2O groups. An additional H2O group, which is not bonded directly to any cation, is located in the interlayer. Along with rabejacite, its Cu-rich variety was found in the specimens examined and characterized structurally. Its crystal structure (R1 = 10.15% for 1049 reflections with I > 3s(I)) is practically the same as that of rabejacite, but there is an additional Cu2+ site located in between pairs of Ca polyhedra. The structural formula is (Ca1.56Cu0.40)Σ1.90[(UO2)4O4(SO4)2](H2O)8, Z = 1. Its existence suggests a greater diversity in zippeite crystal chemistry than was thought previously and also the possibility of a new Cu2+-dominant zippeite mineral besides pseudojohannite.

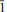 , with a = 8.7434(11), b = 8.309(3), c = 8.8693(10) Å , a = 77.86(2), b = 104.635(11), g = 82.935(18)°, V = 598.8(3) A˚
, with a = 8.7434(11), b = 8.309(3), c = 8.8693(10) Å , a = 77.86(2), b = 104.635(11), g = 82.935(18)°, V = 598.8(3) A˚ 