The exchange of water between plasma and interstitial fluid (ISF) along the length of a capillary is attributed to a balancing of the Starling forces, site-specific differences in hydrostatic and osmotic pressures that theoretically determine directional fluid movement. The osmotic forces for water movement are attributed to the osmotic effects of proteins, colloid osmotic pressure (COP). Several physiological inconsistencies question the role of proteins and COP in fluid flux. A reconsideration of Hulett's insights concerning the osmosis of water provides substantial evidence that the effect of COP does not cause osmosis, and therefore another force is needed to explain plasma–ISF exchange. Review of whole-body tissue and blood ion concentrations and/or ion differences across isolated tissue or secretory epithelia from a variety of species indicates that the diffusion of bicarbonate and strong ions within plasma is the dominant osmotic effect returning ISF to the capillary. Conceptually, as these ions diffuse along physiological gradients, they alter the chemical potential of water through which they are diffusing (solute–solvent drag), creating an osmotic effect on plasma water, and explain plasma–ISF exchange. Considering venous–arterial differences, diffusing 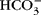 and strong ions give rise to a net osmotic force (~35 Torr) in venous end capillary plasma water that is coupled to ISF through pores in the endothelium. More importantly, diffusing
and strong ions give rise to a net osmotic force (~35 Torr) in venous end capillary plasma water that is coupled to ISF through pores in the endothelium. More importantly, diffusing 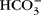 and strong ions provide an incremental osmotic force (~150 Torr) that is essentially matched to any change in metabolic rate (e.g. muscular work) when CO2 output and water production are increased. The proposed diffusing ion osmotic force does not negate the necessity for colloidal proteins in volume regulation. Proteins can have an essential effect on fluid exchange in plasma when blood flow is intermittent or changes in protein concentration in the ISF such that proteins exert a force against a distensible boundary (i.e. the endothelium and basement membrane) as they are reflected by it or diffuse through the membrane due to changes in permeability.
and strong ions provide an incremental osmotic force (~150 Torr) that is essentially matched to any change in metabolic rate (e.g. muscular work) when CO2 output and water production are increased. The proposed diffusing ion osmotic force does not negate the necessity for colloidal proteins in volume regulation. Proteins can have an essential effect on fluid exchange in plasma when blood flow is intermittent or changes in protein concentration in the ISF such that proteins exert a force against a distensible boundary (i.e. the endothelium and basement membrane) as they are reflected by it or diffuse through the membrane due to changes in permeability.

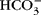 and strong ions give rise to a net osmotic force (~35 Torr) in venous end capillary plasma water that is coupled to ISF through pores in the endothelium. More importantly, diffusing
and strong ions give rise to a net osmotic force (~35 Torr) in venous end capillary plasma water that is coupled to ISF through pores in the endothelium. More importantly, diffusing 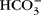 and strong ions provide an incremental osmotic force (~150 Torr) that is essentially matched to any change in metabolic rate (e.g. muscular work) when CO
and strong ions provide an incremental osmotic force (~150 Torr) that is essentially matched to any change in metabolic rate (e.g. muscular work) when CO