The chemistry and crystal structure of a unique Zn-rich kupletskite: (K1.55Na0 .21Rb0.09Sr0.01)Σ1.86(Na0.82Ca0.18)Σ1.00(Mn4.72Zn1.66Na0.41Mg0.12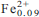 )Σ7.00 (Ti1.85Nb0.11Hf0.03)Σ1.99(Si7.99Al0.12)Σ8.11O26 (OH)4(F0.77OH0.23)Σ1.00, from analkalin e pegmatite at Mont Saint-Hilaire, Quebec, Canada has been determined. Zn-rich kupletskite is triclinic,
)Σ7.00 (Ti1.85Nb0.11Hf0.03)Σ1.99(Si7.99Al0.12)Σ8.11O26 (OH)4(F0.77OH0.23)Σ1.00, from analkalin e pegmatite at Mont Saint-Hilaire, Quebec, Canada has been determined. Zn-rich kupletskite is triclinic,  , a = 5.3765(4), b = 11.8893(11), c = 11.6997(10), α = 113.070(3), β = 94.775(2), γ = 103.089(3), R1 = 0.0570 for 3757 observed reflections with Fo > 4σ(Fo). From the single-crystal X-ray diffraction refinement, it is clear that Zn2+ shows a preference for the smaller, trans M(4) site (69%), yet is distributed amongst all three octahedral sites coordinated by 4 O2− and 2 OH− [M(2) 58% and M(3) 60%]. Of note is the lack of Zn in M(1), the larger and least-distorted of the four crystallographic sites, with an asymmetric anionic arrangement of 5 O2− and 1 OH−. The preference of Zn for octahedral sites coordinated by mixed ligands (O and OH) is characteristic of its behaviour in alkaline systems, in contrast to granitic systems where Zn tends to favour [4]-coordinated, OH− and H2O-free sites with only one ligand species (O, S, Cl, B, I). In alkaline systems, [4]Zn is only present in early sphalerite or in late-stage zeolite-like minerals. The bulk of Zn in alkaline systems is present as discrete [6]Zn phases such as members of the astrophyllite, labuntsovite, milarite and nordite groups, a result of the formation of network-forming
, a = 5.3765(4), b = 11.8893(11), c = 11.6997(10), α = 113.070(3), β = 94.775(2), γ = 103.089(3), R1 = 0.0570 for 3757 observed reflections with Fo > 4σ(Fo). From the single-crystal X-ray diffraction refinement, it is clear that Zn2+ shows a preference for the smaller, trans M(4) site (69%), yet is distributed amongst all three octahedral sites coordinated by 4 O2− and 2 OH− [M(2) 58% and M(3) 60%]. Of note is the lack of Zn in M(1), the larger and least-distorted of the four crystallographic sites, with an asymmetric anionic arrangement of 5 O2− and 1 OH−. The preference of Zn for octahedral sites coordinated by mixed ligands (O and OH) is characteristic of its behaviour in alkaline systems, in contrast to granitic systems where Zn tends to favour [4]-coordinated, OH− and H2O-free sites with only one ligand species (O, S, Cl, B, I). In alkaline systems, [4]Zn is only present in early sphalerite or in late-stage zeolite-like minerals. The bulk of Zn in alkaline systems is present as discrete [6]Zn phases such as members of the astrophyllite, labuntsovite, milarite and nordite groups, a result of the formation of network-forming 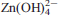 complexes inthe low-temperature, low-fS2, high-alkalinity and highly oxidizing systems.
complexes inthe low-temperature, low-fS2, high-alkalinity and highly oxidizing systems.

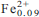 )
)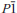 ,
, 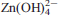 complexes inthe low-temperature, low-
complexes inthe low-temperature, low-