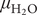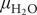The chemical potential of water (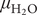 $ {\mu}_{{\mathrm{H}}_2\mathrm{O}} $) provides an essential thermodynamic characterization of the environment of living organisms, and it is of equal significance as the temperature. For cells,
$ {\mu}_{{\mathrm{H}}_2\mathrm{O}} $) provides an essential thermodynamic characterization of the environment of living organisms, and it is of equal significance as the temperature. For cells, 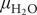 $ {\mu}_{{\mathrm{H}}_2\mathrm{O}} $ is conventionally expressed in terms of the osmotic pressure (πosm). We have previously suggested that the main contribution to the intracellular πosm of the bacterium E. coli is from soluble negatively-charged proteins and their counter-ions. Here, we expand on this analysis by examining how evolutionary divergent cell types cope with the challenge of maintaining πosm within viable values. Complex organisms, like mammals, maintain constant internal πosm ≈ 0.285 osmol, matching that of 0.154 M NaCl. For bacteria it appears that optimal growth conditions are found for similar or slightly higher πosm (0.25-0.4 osmol), despite that they represent a much earlier stage in evolution. We argue that this value reflects a general adaptation for optimising metabolic function under crowded intracellular conditions. Environmental πosm that differ from this optimum require therefore special measures, as exemplified with gram-positive and gram-negative bacteria. To handle such situations, their membrane encapsulations allow for a compensating turgor pressure that can take both positive and negative values, where positive pressures allow increased frequency of metabolic events through increased intracellular protein concentrations. A remarkable exception to the rule of 0.25-0.4 osmol, is found for halophilic archaea with internal πosm ≈ 15 osmol. The internal organization of these archaea differs in that they utilize a repulsive electrostatic mechanism operating only in the ionic-liquid regime to avoid aggregation, and that they stand out from other organisms by having no turgor pressure.
$ {\mu}_{{\mathrm{H}}_2\mathrm{O}} $ is conventionally expressed in terms of the osmotic pressure (πosm). We have previously suggested that the main contribution to the intracellular πosm of the bacterium E. coli is from soluble negatively-charged proteins and their counter-ions. Here, we expand on this analysis by examining how evolutionary divergent cell types cope with the challenge of maintaining πosm within viable values. Complex organisms, like mammals, maintain constant internal πosm ≈ 0.285 osmol, matching that of 0.154 M NaCl. For bacteria it appears that optimal growth conditions are found for similar or slightly higher πosm (0.25-0.4 osmol), despite that they represent a much earlier stage in evolution. We argue that this value reflects a general adaptation for optimising metabolic function under crowded intracellular conditions. Environmental πosm that differ from this optimum require therefore special measures, as exemplified with gram-positive and gram-negative bacteria. To handle such situations, their membrane encapsulations allow for a compensating turgor pressure that can take both positive and negative values, where positive pressures allow increased frequency of metabolic events through increased intracellular protein concentrations. A remarkable exception to the rule of 0.25-0.4 osmol, is found for halophilic archaea with internal πosm ≈ 15 osmol. The internal organization of these archaea differs in that they utilize a repulsive electrostatic mechanism operating only in the ionic-liquid regime to avoid aggregation, and that they stand out from other organisms by having no turgor pressure.