Dolomites form in a range of environments by processes able to drive large volumes of magnesium-rich waters through existing carbonate sediments or rocks. These fluids need not be of unusual composition but there are kinetic barriers to crystallisation which is influenced by factors such as the Mg/Ca ratio, salinity, temperature, the 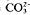 /Ca2+ ratio and time. Dolomite is able to form at near-surface temperatures and pressures within a few thousand years.
/Ca2+ ratio and time. Dolomite is able to form at near-surface temperatures and pressures within a few thousand years.
Textures in dolomitic rocks are controlled by their conditions of formation. A large proportion are replacive but few of these are mimetic, preserving primary structures. Crystals vary from euhedral to anhedral with boundaries ranging from planar to consertal. Solution chemistry and temperatures influence the density and distribution of nuclei together with growth rates and crystal morphology. There is still doubt whether irregular crystal faces are products of high or low temperatures or saturation. Dolomite cements are more important than has previously been realised in massively dolomitised rocks. Differential dissolution of aragonite, calcite, or evaporite minerals leaves space for these cements to occupy. Dolomitisation may also be allied to compaction, generating stylolitic rocks which are progressively enriched in dolomite. Dolomite may be replaced by calcite or it may be dissolved and the resulting pores filled with a calcite cement.
There is no general correlation between any set of petrographic features and particular geological models for dolomitisation. Similar physicochemical conditions are reproduced in a range of environments and the most effective guides to origin are in the geometry and regional petrographic variation of dolomite bodies.

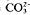 /Ca
/Ca