Crystallite growth in natural agate samples has been investigated at temperatures of 350—550°C and 100 MPa pressure in the presence of water vapour. Initial crystallite coarsening is accompanied by the transformation of moganite to α-quartz that is apparently inhibited by residual moganite when the crystallite sizes reach ~50 nm. At 350—500°C the coarsening kinetics can be described by an empirical law developed to describe Zener pinning which incorporates the maximum crystallite size prior to growth inhibition: 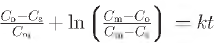 . Co = initial crystallite size, Cs = crystallite growth after time t, Cm = the maximum size achieved before inhibition and k is the rate constant that includes the activation energy which was found to be 51(±9) kJ mole—1. A more conventional isothermal growth rate law,
. Co = initial crystallite size, Cs = crystallite growth after time t, Cm = the maximum size achieved before inhibition and k is the rate constant that includes the activation energy which was found to be 51(±9) kJ mole—1. A more conventional isothermal growth rate law, 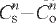 = kt with n = 6.5, only applies at 550°C. Limited growth was obtained when small agate cubes were heated in an open furnace up to 122 d at 550°C, demonstrating that water vapour was essential for continued crystallite coarsening. The crystallite size and moganite content of agates formed under normal earth surface conditions from hosts aged 13 Ma to 3.5 Ga have also been determined. The high temperature crystallite growth rate law does not describe natural agate growth quantitatively but a qualitatively similar pattern is observed.
= kt with n = 6.5, only applies at 550°C. Limited growth was obtained when small agate cubes were heated in an open furnace up to 122 d at 550°C, demonstrating that water vapour was essential for continued crystallite coarsening. The crystallite size and moganite content of agates formed under normal earth surface conditions from hosts aged 13 Ma to 3.5 Ga have also been determined. The high temperature crystallite growth rate law does not describe natural agate growth quantitatively but a qualitatively similar pattern is observed.

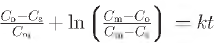 .
. 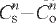 =
= 