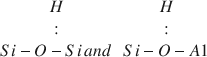The infrared absorption spectra of a palygorskite sample from Cáceres, Spain, showed two previously unreported bands in the OH-stretching region at 3420–3440 and 3220–3230 cm−1 after evacuation at 90°–230°C. These bands, which reached maximum intensity after the sample was heated at 150°C, were assigned to OH in the$$\begin{array}{*{20}{c}} H \\: \\ {Si - O - Si\,and\,} \\ \end{array}\begin{array}{*{20}{c}} H \\: \\ {Si - O - A1} \\ \end{array}$$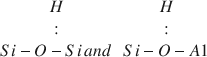
groups, respectively. To characterize the nature of these OH groups, pyridine was adsorbed on the sample. The resultant spectra suggest that at 150°C the palygorskite folded and OH groups protonated, resulting in the formation of a deformed pyridinium ion between 150° and 290°C. A high concentration of thermally stable Lewis-acid sites on the surface of the palygorskite was also noted.