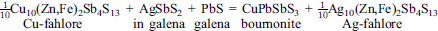The sulphide ores from the Julcani mining district (Peru) display many retrograde reactions that may be attributed to solid-state processes accompanying cooling. Fahlores [˜(Cu,Ag)10(Zn,Fe)2(Sb,As)4S13] from the Herminia mine exhibit pronounced downstream enrichments in molar Ag/(Ag+Cu) ratios that are strongly correlated with the abundance of PbS-AgSbS2-AgBiS2 phases. These correlations, discontinuous core to rim Sb/(Sb+As) enrichments in bournonites, and prominent reaction textures involving fahlores, bournonites and galenas provide strong evidence that the fahlores in these ores have been enriched in Ag by the Ag–Cu exchange reaction
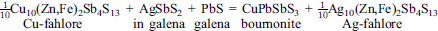
which occurred during cooling following mineralization of a PbS-AgSbS2-AgBiS2 galena and has been documented elsewhere. Secondary PbS-AgSbS2-AgBiS2 minerals aramayoite, bismuthian diaphorite [Pb2Ag3(Bi,Sb)3S8], and diaphorite were produced from primary galenas with cooling of ores with high Pb/Cu and Bi/Sb; pyrargyrite formed from the breakdown of the Ag10Zn2Sb4S13 component in the most Ag-rich fahlores, as an exsolution product of galena, and from replacement of aramayoite and galena with the evolution of semimetal sulphides. Based on mineral compositions, phase equilibria, a thermochemical database for sulphides and sulphosalts, and the reintegrated composition for primary grains of Ag-rich PbS-AgSbS2-AgBiS2 phases, we estimate that the primary temperature of hydrothermal mineralization was >320±10°C, that these reactions ceased to affect fahlore Ag/(Ag+Cu) ratios and Bi/(Bi+Sb) ratios of aramayoite and miargyrite after cooling through 220±10°C. Galenas, however, appear to have continued to adjust their compositions to reflect even lower temperatures by continued exsolution of semimetals and production a diverse suite of sulphosalts that occur in fine intergrowths with galena.