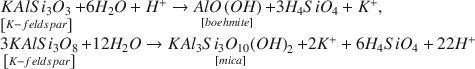Experimental alterations of K-feldspar in distilled-deionized water at 150°, 175°, 200°, and 225°C were performed. The alteration products and dissolution mechanism of K-feldspar were examined by X-ray powder diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray analysis (EDX), and X-ray photoelectron spectroscopy (XPS). SEM, TEM, and EDX clearly showed formation of fibrous boehmite less than 1.0 μm in length at the early alteration stages. The boehmite fibers decreased in abundance and rounded platy 1 M mica was produced as alteration proceeded. The mica exhibited initially angular shaped small flakes of 0.69 μm in average size, which developed to rounded platy particles of 1.97 μm. The main chemical reactions occurring in this experimental system can be expressed by: $$\begin{array}{l} \mathop {KAlS{i_3}{O_3}}\limits_{\left[ {K - feldspar} \right]} + 6{H_2}O + {H^ + } \to \mathop {AlO\left( {OH} \right)}\limits_{\left[ {boehmite} \right]} + 3{H_4}Si{O_4} + {K^ + }, \\ \mathop {3KAlS{i_3}{O_8}}\limits_{\left[ {K - feldspar} \right]} + 12{H_2}O \to \mathop {KA{l_3}S{i_3}{O_{10}}{{\left( {OH} \right)}_2}}\limits_{\left[ {mica} \right]} + 2{K^ + } + 6{H_4}Si{O_4} + 22{H^ + }. \\ \end{array}$$ XPS showed no significant changes in intensities of photoelectron lines excited from K, Si, and Al in the K-feldspar surface before and after alteration, however the K/Si molar ratios in the solutions were considerably smaller than that of the original K-feldspar. The results of XPS strongly indicate that no dealkalized layer was produced on the surface, and that dissolution of K-feldspar in aqueous solution proceeded congruently by a surface-reaction mechanism. The discrepancy of mass balance in the solutions may be mainly caused by adsorption of K on the surface of boehmite.
XPS showed no significant changes in intensities of photoelectron lines excited from K, Si, and Al in the K-feldspar surface before and after alteration, however the K/Si molar ratios in the solutions were considerably smaller than that of the original K-feldspar. The results of XPS strongly indicate that no dealkalized layer was produced on the surface, and that dissolution of K-feldspar in aqueous solution proceeded congruently by a surface-reaction mechanism. The discrepancy of mass balance in the solutions may be mainly caused by adsorption of K on the surface of boehmite.