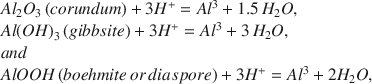The solubilities of HCl-treated samples of corundum, gibbsite, boehmite, and diaspore in aqueous solutions at 298 K and one atmosphere pressure were determined from undersaturated and supersaturated initial conditions. Solution characteristics at apparent equilibrium were measured and used to calculate equilibrium constants (Kr) for mineral dissolution reactions:$$\begin{array}{l} A{l_2}{O_3}\left( {corundum} \right) + 3{H^ + } = A{l^3} + 1.5\,{H_2}O,\\ Al{\left( {OH} \right)_3}\left( {gibbsite} \right) + 3{H^ + } = A{l^3} + 3\,{H_2}O,\\ and\\ AlOOH\left( {boehmite\,or\,diaspore} \right) + 3{H^ + } = A{l^3} + 2{H_2}O, \end{array}$$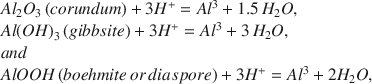
Assuming solid phase and water activities of unity, log Kr = 3pH - pAl3+ for all three equations. The calculated log Kr values were: 9.01 ± 0.05 (corundum), 7.76 ± 0.14 (gibbsite), 7.49 ± 0.09 (boehmite), and 6.75 ± 0.24 (diaspore), indicating that the relative thermodynamic stabilities under the experimental conditions were: corundum < gibbsite < boehmite < diaspore. The gibbsite value agreed well with that determined independently by another research group using acid-treated subsamples of the same source mineral (7.70 ± 0.02). The calculated Gibbs free energies of formation (kJ/mole) were: -1587.4 ± 2.1 (corundum), -1156.7 ± 1.6 (gibbsite), -921.0 ± 1.5 (boehmite), and -925.1 ± 2.0 (diaspore). The Δ Gfo values of the acid-treated minerals were 1.8 to 12.4 kJ/mole more negative than values recently compiled for untreated mineral samples. This result is attributed to the removal of reactive surface coatings that isolated the bulk mineral phases from the solution phase.