INTRODUCTION
Non-typhoidal Salmonella is estimated to be the most common cause of bacterial foodborne illnesses in the United States, causing an estimated one million foodborne illnesses and about 130 outbreaks annually [Reference Scallan1, 2]. Despite efforts to prevent Salmonella contamination of foods, the incidence of Salmonella infections, as reported by the Foodborne Diseases Active Surveillance Network (FoodNet), did not decrease between 1996 and 2011 [3]. An analysis based on outbreak data from 1998 to 2008 estimated beef to be the third most common source of US bacterial foodborne illness and the fourth most common source of Salmonella outbreaks attributed to a commodity after poultry, eggs, and pork [Reference Gould4]. Recent, large outbreaks of Salmonella, including some caused by multidrug-resistant strains, have highlighted the role of beef, particularly ground beef, as an important source of foodborne infections [5–Reference McLaughlin7].
Cattle are known reservoirs of Salmonella, which can contaminate meat during slaughter and subsequent processing steps [Reference Roels8]. In 1998, the United States Department of Agriculture's Food Safety and Inspection Service (USDA-FSIS) implemented a beef carcass and ground beef Salmonella testing programme to verify that establishments were maintaining process control and were meeting performance standards [9]. Testing by USDA-FSIS during the 2000s showed <3% of ground beef samples are positive for Salmonella, in contrast to 7·5% in 1993–1994 [9, 10]. Each year in the United States, over 26 billion pounds of beef are consumed [11], and in one survey, 18% of persons who reported consuming ground beef in the previous 7 days indicated that it was raw or undercooked [Reference Taylor12]. Based on a court ruling, USDA-FSIS cannot consider Salmonella to be an adulterant of raw beef because the product is expected to be handled properly and adequately cooked before consumption, thereby destroying pathogens [13]. In select situations where illnesses are linked to a raw meat, FSIS has made determinations that the product was adulterated and the product was recalled by the processor [14]. Salmonella outbreaks attributed to beef have not been previously described. In this report, we describe and analyse salmonellosis outbreaks in the United States that were attributed to beef and reported from 1973 to 2011.
METHODS
State, local, and territorial health departments have voluntarily submitted reports of foodborne disease outbreak investigations to the CDC's Foodborne Disease Outbreak Surveillance System (FDOSS) (www.cdc.gov/foodsafety/fdoss) since 1973. We queried FDOSS for reports of outbreaks of Salmonella infections from 1973 to 2011 in which beef was listed as the implicated food vehicle. We also searched Morbidity and Mortality Weekly Reports (MMWR), along with the EMBASE, Medline, and Web of Science databases using the following key words: salmonella, salmonella food poisoning/infection/outbreak, and beef; an additional six outbreaks that occurred between 1975 and 1996 were identified and were included in the analysis [15–Reference Eidson18].
A foodborne disease outbreak is defined by CDC as the occurrence of two or more cases of a similar illness resulting from the ingestion of a common food. Outbreaks were included in the analysis if Salmonella was the only reported aetiology and if the implicated food was beef or a food containing beef, like tacos, where the contaminated ingredient was identified as beef.
Since 2003, CDC's National Antimicrobial Resistance Monitoring System (NARMS) laboratory has performed antimicrobial susceptibility tests on representative clinical isolates from outbreak investigations [19]. Antimicrobial susceptibility results from NARMS were included in the analysis when available. Additionally, the results of antimicrobial susceptibility testing were included from the published literature for six outbreaks [5–Reference Roels8, Reference Fontaine20, Reference Spika21]. Clinical and Laboratory Standards Institute (CLSI) interpretive criteria were used when available [19].
Variables included in outbreak reports and used in descriptive analyses included number of outbreaks, illnesses, and hospitalizations, patient demographics (age, gender), non-typhoidal Salmonella species and serotype, month and year of outbreak, food preparation settings, factors contributing to food contamination (complete list of factors available at http://www.cdc.gov/nors/pdf/NORS_Guidance_5213-508c.pdf), traceback investigations, and products recalled. Multiple food preparation settings could be reported; all preparation settings reported in each outbreak were included in the descriptive analysis. We classified the beef products implicated into four mutually exclusive categories: roast beef, ground beef, other beef types and unknown beef type.
Contributing factors were grouped into five categories according to whether they represented contamination of raw ingredients or foods before food preparation (environmental contamination), contamination or amplification during food preparation and processing, such as inadequate time or temperature during cooking (improper food handling), direct contamination of food by a food handler who was ill or a carrier of the pathogen (worker contamination), cross-contamination in the food preparation environment (cross-contamination), or other contamination factors (other contamination). More than one contributing factor could be reported. All contributing factors reported in each outbreak were included in the descriptive analysis.
Statistical tests were performed using non-parametric methods when sample size was sufficient, including Wilcoxon rank-sum test and Fisher's exact χ 2 test. Statistical analyses were performed using SAS v. 9·3 (SAS Institute Inc., USA).
RESULTS
From 1973 to 2011, 28 599 foodborne outbreaks were reported to CDC. Of the 1965 outbreaks reported to CDC where Salmonella was the aetiological agent and a food vehicle was implicated, beef was the implicated food vehicle in 90 outbreaks. Including the six additional outbreaks reported in the literature, 96 beef-attributed salmonellosis outbreaks in total were identified. These 96 outbreaks accounted for 3684 illnesses, 318 hospitalizations, and five deaths (Table 1). All outbreaks were due to Salmonella enterica. A serotype was reported for 89 (93%) outbreaks (Table 2). Thirty serotypes caused outbreaks, most commonly Typhimurium (16 outbreaks, 17%), Newport (15, 16%), and Enteritidis (9, 9%). Outbreaks caused by serotypes Newport and Typhimurium also accounted for more illnesses (656, 18%; 614, 17%) and hospitalizations (93, 29%; 58, 18%), respectively, than any other single serotype. A median of two outbreaks (range 0–9) and 63 outbreak-related illnesses (range 0–200) were reported each year. No changes over time in the number of outbreaks reported annually were apparent (Fig. 1). The median size of these outbreaks was 29 illnesses (range 2–200) (Table 1). The largest outbreak reported to CDC, a multistate outbreak of 200 Salmonella serotype Newport infections associated with roast beef, occurred in 1977 [22].

Fig. 1. Outbreaks of Salmonella enterica infections where beef was the implicated vehicle, by type and year, United States, 1973–2011.
Table 1. Characteristics of outbreaks of Salmonella enterica infections where beef was the implicated food vehicle, by beef type, United States, 1973–2011

* Eight types of beef were reported: steak (8 outbreaks); brisket and jerky (7 outbreaks each); barbecued (4); barbacoa, beef blood, ribs, tripe (1 each).
† Gender data collected since 1998; information available for 36 (78%) outbreaks.
‡ Age data collected since 1998; age distribution available for 33 (72%) outbreaks.
§ Not mutually exclusive; 88 (92%) outbreak reports included data on at least one food preparation setting (24 roast beef, 19 ground beef, 29 other type, 17 unknown beef type); 69 outbreaks reported only one setting (20 roast beef, 12 ground beef, 22 other type, and 15 unknown beef type).
¶ Other or unspecified setting: unspecified (11 reports); school (5); cafeteria or hall, caterer, supermarket or grocery store (4 each); church or temple, fair, festival, or other temporary mobile food service (2 each); picnic, field dressed cow (1 each).
|| Not mutually exclusive; data on contributing factors were collected beginning in 1998. At least one contributing factor was reported for 31 (67%) of the 46 outbreaks (4 roast beef, 17 ground beef, 16 other, and 9 unknown) reported during 1998 to 2011.
Table 2. Characteristics of outbreaks of Salmonella enterica infections in which beef was the implicated vehicle, by serotype, United States, 1973–2011
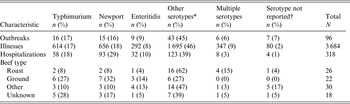
* The other serotypes were Heidelberg, Montevideo, Saintpaul (4 outbreaks each); Berta, Infantis, Thompson (3 outbreaks each); Agona, Anatum, Chester, Hadar, Reading (2 outbreaks each); Agama, Bovismorbificans, Braenderup, Cerro, Derby, Hartford, Kiambu, Ohio, Oranienburg, Singapore, Schwarzengrund, Senftenberg (1 outbreak each).
† Serogroup was reported in three outbreaks with no serotype reported, including Group B (2 outbreaks) and Group C1 (1 outbreak).
Beef types implicated in outbreaks
Roast beef was implicated in 26 (27%) outbreaks, ground beef in 22 (23%), and other types in 30 (31%); the type was not specified in the remaining 18 (19%). In 13 (93%) of 14 roast beef-attributed outbreaks with information available on the preparation of the roast beef, the beef was pre-cooked, delicatessen-style. Steak (n = 8 outbreaks), jerky (n = 7), and brisket (n = 7) were the types most commonly implicated in outbreaks in the other types of beef category. Salmonella serotypes Typhimurium and Newport accounted for over half (59%) of the ground beef-attributed outbreaks, and Typhimurium accounted for 28% of outbreaks caused by unknown beef type, whereas no serotype predominated in outbreaks attributed to roast beef or other types of beef. Outbreaks differed in size by type of beef (median 47 illnesses per outbreak for roast, 36 for ground, 15 for other types, and 19 for unknown types) (Table 1). For 36 outbreaks with information available on the gender and age of patients, the percentage of patients who were female did not differ significantly by beef type (Wilcoxon rank sum test, P = 0·53); overall, 52% of patients were female. Differences in the age distribution of patients by type of beef were not apparent; overall, 50% were aged 20–49 years (range by type 44–59%).
Roast beef-attributed outbreaks were more common in the first half of the surveillance period; 21 (81%) of 26 roast beef-attributed outbreaks were reported during 1973–1991. In contrast, ground beef emerged as the predominant type in 2002; 17 (77%) of 22 ground beef-attributed outbreaks were reported during 2002–2011. In addition, ground beef was responsible for 17 (45%) of 38 outbreaks reported during 2002–2011 (Fig. 1). The distribution of ground beef- and roast beef-attributed outbreaks pre-1993 vs. post-1993 was significantly different (Fisher's exact test, P < 0·0001). Outbreaks attributed to other types of beef were first reported in 1985 and occurred throughout the study period.
The number of outbreaks reported varied by month, increasing in May, reaching a peak in August, and dropping sharply in November (Fig. 2). With one exception, roast beef-attributed outbreaks were reported during April–September. The majority of ground beef-attributed outbreaks were reported during May–October. Outbreaks were reported throughout the year for other or unknown beef type.

Fig. 2. Distribution of beef-attributed outbreaks of Salmonella enterica infections, by type and month, United States, 1973–2011.
Geography of and multistate outbreaks attributed to beef
Outbreaks occurred in 46 states and Washington, DC, with the most reported from New York (18 outbreaks), California (14) and Wisconsin (10) (Fig. 3). Traceback investigations were performed in six outbreaks; two outbreaks, in 2002 and 2003, were traced back to the same large slaughterhouse [Reference Talbot, Gagnon and Greenblatt23]. Of the six outbreaks in which traceback investigations were performed, product was recalled in one; product was also recalled in an additional three outbreaks.
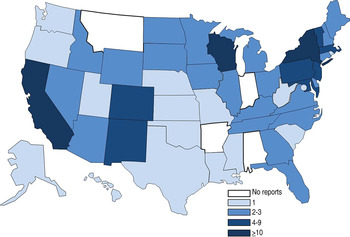
Fig. 3. Number of Salmonella enterica outbreaks where beef was the implicated vehicle by state, 1973–2011. States were involved in 85 single-state and 11 multistate outbreaks. Multistate outbreaks are counted as an outbreak for each state that reported a case. [States involved in multistate outbreaks included: Arizona (2 multistate outbreaks), California (2), Colorado (3), Connecticut (3), Delaware (1), Idaho (2), Illinois (1), Iowa (1), Kansas (1), Kentucky (1), Maine (1), Maryland (2), Massachusetts (2), Michigan (2), Nebraska (1), Nevada (2), New Hampshire (1), New Jersey (5), New Mexico (2), New York (5), Pennsylvania (5), Rhode Island (1), South Dakota (2), Tennessee (2), Texas (1), Utah (1), Vermont (1), Virginia (1), Wisconsin (1), Wyoming (2), Washington, D.C. (1).]
Eleven multistate outbreaks were reported, involving a median of four states reporting cases (range 2–15) and were caused by serotypes Newport (6 outbreaks), Typhimurium (3), Berta (1) and Bovismorbificans (1). Ground beef was implicated in 9 (82%) of 11 multistate outbreaks, all but two of which occurred after 2001; two were linked to roast beef and occurred in 1976 and 1977. Although multistate outbreaks accounted for only 11% of reported outbreaks and 19% (697) of illnesses, they accounted for a disproportionately larger number of multidrug-resistant outbreaks (11, 79%), hospitalizations (93, 29%), and deaths (2, 40%) than single state outbreaks.
Preparation settings reported in outbreaks
Reports of 88 (92%) outbreaks included data on settings where food was prepared (Table 1). The most common overall were restaurants or delicatessens (35, 40%) and private homes (25, 28%). In outbreaks involving roast beef, the most common preparation settings were restaurants or delicatessens (10, 42%, all but three during 1975–1988) and processing plants (9, 38%, the last identified in 1986); only one roast beef-attributed outbreak report indicated a private home as the preparation setting. By contrast, the most common food preparation setting in outbreaks involving ground beef was a private home (12, 63%).
Contributing factors reported for outbreaks
At least one contributing factor was reported for 31 (67%) of the 46 outbreaks reported during 1998–2011 (Table 1). Improper food handling (67%) and environmental contamination (61%) were the most common, followed by cross-contamination (45%), worker contamination (23%), and other contamination (6%). Environmental contamination was reported in over half the ground beef-attributed outbreaks (59%) but not in any roast beef-attributed outbreaks. Conversely, cross-contamination was reported in three (75%) of the four roast beef-attributed outbreaks vs. two (12%) of 17 ground beef-attributed outbreaks. The most commonly reported contributing factor for outbreaks attributed to other beef types was improper food handling (50%).
Antimicrobial susceptibility results for outbreak strains
Antimicrobial resistance data were available for 14 outbreaks. Strains from six outbreaks were susceptible to all agents tested and were caused by serotypes Typhimurium (2 outbreaks), Anatum (1), Berta (1), Montevideo (1), Newport (1); the beef types were ground (3 outbreaks), roast (2) and other: barbecued (1). Strains from the other eight outbreaks were resistant to at least three classes of antimicrobial agents; all these outbreaks were due to ground beef. Five were caused by serotype Newport, including two resistant to ampicillin, chloramphenicol, streptomycin, sulfamethoxazole/sulfisoxazole, tetracycline, amoxicillin-clavulanic acid, ceftriaxone (ACSSuTAuCx) and one only resistant to ACSSuT (ampicillin, chloramphenicol, streptomycin, sulfamethoxazole/sulfisoxazole, tetracycline); three were caused by serotype Typhimurium, including one resistant to ACSSuTAuCx and one only resistant to ACSSuT. The median percentage of patients hospitalized was higher in outbreaks caused by resistant strains (median 23%, range 4–40), than in outbreaks caused by strains with no resistance detected (10%, range 8–26), although the difference was not significant (Wilcoxon rank test, P = 0·6).
DISCUSSION
Our analysis highlights that for 38 years, beef-attributed outbreaks of salmonellosis have occurred regularly in the United States at a rate of about two per year, despite the fact that consumption of beef in the United States dropped 34% during the same time period [24]. Although roast beef and ground beef were implicated in a similar number of outbreaks during this period, we observed a marked change in the type of beef implicated over time. Most of the roast beef-attributed outbreaks occurred during 1973–1992, while nearly all ground beef-attributed outbreaks occurred during the last 10 years (2002–2011).
Roast beef-attributed outbreaks, caused by a variety of Salmonella serotypes, were predominant during the 1970s and 1980s. During these years, the implicated roast beef was typically delicatessen-style, pre-cooked at a processing establishment. After several outbreak investigations in the 1970s revealed that the cooking temperature required to eliminate Salmonella was often not achieved during processing [Reference Shapiro17, 25, Reference Parham26], USDA-FSIS passed a series of regulations to ensure proper cooking of commercially pre-cooked roast beef [25–27], including an emergency change in 1977 requiring that roast beef be heated throughout to 145 °F (62·8 °C) [25, Reference Parham26]. USDA-FSIS amended this requirement in 1978 to cater for consumers' preference for roast beef that appeared rare or pink by providing alternative cooking times and temperatures that maintain the appearance of rare roast beef but eliminate Salmonella [Reference Parham26]. After learning that humidity was not well-controlled during cooking, although moist heat is more effective than dry heat in killing Salmonella, USDA-FSIS set further standards for production facilities, including guidance on humidity during cooking [27]. The roast beef-attributed outbreaks that occurred during the years immediately following passage of the new regulations resulted from failures to comply with regulations regarding roast beef processing and storage temperature [25, 28, Reference Spitalny, Okowitz and Vogt29]. However, the long-term positive impact of the regulations is clear. The last reported outbreak attributed to roast beef prepared in a commercial processing plant occurred in 1986.
Ground beef emerged as a predominant food vehicle in outbreaks reported during the 2000s. Several factors are hypothesized to have contributed to this emergence, including increased exposure to contaminated beef through changes in consumer and industry practices, and increased detection of outbreaks. Although average annual per capita consumption of ground beef declined from 33·7 pounds in 1980 to 27·6 pounds in 2006 [30], a population-based survey by USDA-FSIS and the U.S. Food and Drug Administration noted a significant increase in the percentage of persons who consumed undercooked ground beef during the 2000s [Reference Fein31]. In addition, the beef processing industry has become increasingly consolidated and complex. The percentage of beef produced by the four largest US firms operating large meat-processing plants increased from 26% in 1972 to 80% in 1997 [Reference Kandel and Parrado32]; large plants like these often mix and grind beef from multiple carcasses [Reference Armstrong, Hollingsworth and Morris33]. These circumstances provide an opportunity for contaminated beef from a single animal or supplier to be dispersed throughout a large batch of ground beef that is then widely distributed [Reference Armstrong, Hollingsworth and Morris33]. Variations in contamination of beef due to industry changes could have influenced the number of outbreaks. Although studies and sampling programmes have varied over time, one USDA survey in 1975 reported that only 0·4% of raw beef patties sampled were contaminated with Salmonella [Reference Surkiewicz34], whereas USDA-FSIS reported an estimated 7·5% prevalence in ground beef samples in 1993–1994 [10] and <3% in the 2000s [9]. In addition, proactive decisions by USDA-FSIS in ground beef outbreak investigations might have also contributed to the increase. For example, in December 2007, USDA-FSIS issued a public health alert regarding ground beef products sold by a supermarket chain related to an outbreak of multidrug-resistant serotype Newport infections [35]. In July 2009, USDA-FSIS announced a recall of over 450 000 pounds of ground beef related to an outbreak of infections with serotype Typhimurium DT104 infections [35]. In the 6 December 2012 Federal Register USDA-FSIS stated, ‘When NRTE poultry or meat products are associated with an illness outbreak and contain pathogens that are not considered adulterants, FSIS likely will consider the product linked to the illness outbreak to be adulterated under 21 U.S.C. 453(g)(3) or 21 U.S.C. 601(m)(3) because the product is “unsound, unhealthful, unwholesome, or otherwise unfit for human food” ’ [14]. It is also likely that some multistate outbreaks were occurring before the 2000s but were not detected. CDC PulseNet, the national molecular subtyping network, identifies strains with similar pulsed-field gel electrophoresis (PFGE) patterns, assisting epidemiologists and other public health professionals in identifying and investigating widely dispersed outbreaks (http://www.cdc.gov/pulsenet/index.html) [Reference Ribot36]. Although established in 1996, national participation in PulseNet for Salmonella isolates was not reached until 2001, and the number of Salmonella isolates subtyped increased almost ninefold between 2001 and 2012 (Kelley Hise, CDC, personal communication), while the incidence of laboratory-confirmed Salmonella infections in U.S. FoodNet sites remained unchanged [3]. Increased participation in PulseNet is the most likely reason that reports of multistate outbreaks, including those attributed to beef, have been increasing [Reference Gould4].
The percentage of outbreaks caused by the serotypes most commonly isolated from humans during the study period (Typhimurium and Enteritidis) [37] was relatively low for roast beef (12%) and other types of beef (24%) but 41% for ground beef and 33% for unknown type of beef. This, plus the finding that most ground beef-attributed outbreaks occurred in private homes, and that outbreaks of foodborne illness in private homes are generally less recognized than those at restaurants [Reference Gould4], raises the possibility that ground beef is responsible for an even greater proportion of Salmonella illnesses than would be expected from an analysis of outbreak data [Reference Gould4].
Serotypes Newport and Typhimurium were the only serotypes identified in outbreaks caused by a multidrug-resistant strain. Multidrug-resistant strains of these serotypes emerged over the past three decades [Reference Greene38, Reference Varma39]. Dairy cattle are an important source of both serotypes, particularly multidrug-resistant strains, and beef cattle might also be an important source of multidrug-resistant serotype Typhimurium strains [5, Reference Greene38]. One study found that US regions with the highest density of dairy cattle were also the regions where multidrug-resistant serotype Newport strains were isolated more frequently from ill persons [Reference Greene38]. The same relationship was observed between beef cattle density and multidrug-resistant serotype Typhimurium infections [Reference Greene38].
Dairy cattle at the end of their milk-producing careers are a source of leaner meat than beef cattle and are commonly used for ground beef [5]; they might be ill or stressed and consequently more likely to be shedding Salmonella [Reference Call, Davis and Sawant40]. Further studies are also needed to understand how food animal production practices, including antimicrobial use, as well as animal age and health status might affect contamination of beef products.
This report has additional limitations. Even when outbreaks are reported, the vehicle or the contaminated ingredient is not always identified. Not all outbreaks are identified, and not all identified outbreaks are investigated or reported to CDC. Other outbreaks might have been due to beef, but beef might not be implicated if it was an ingredient, e.g. in beef stew. The contributing factors reported might be subjective observations because they are made at the time of the investigation and therefore might not have actually contributed to the outbreak. For example, investigators may have listed a restaurant or delicatessen as the point of preparation for contaminated roast beef inadequately cooked in a processing plant. The small proportion of outbreaks where information was available to pursue a traceback investigation (6%) limited our ability to assess factors contributing to contamination and amplification along the farm-to-table continuum. Finally, antimicrobial susceptibility testing was not assessed for isolates from most outbreaks, so those findings cannot be generalized. It is likely that strains were tested from certain outbreaks because resistance was suspected (e.g. outbreaks caused by serotype Newport) or because the outbreak was multistate. In addition, antimicrobial susceptibility testing methods and interpretations likely varied between laboratories.
In 1996, USDA-FSIS issued Pathogen Reduction; Hazard Analysis and Critical Control Point (PR/HACCP) Systems; Final Rule, which aimed at preventing foodborne illness caused by FSIS-regulated products [41]. The rule included Salmonella performance standards for ground beef and encouraged the industry to implement measures to reduce the contamination of meat and poultry. The initial expansion of the use of pathogen reduction measures, such as thermal and chemical carcass decontamination, steam vacuuming, and steam pasteurization [Reference Stopforth and Sofos42], likely contributed to the initial decreases in Salmonella contamination of ground beef. However, in one recent study, Salmonella, including multidrug-resistant strains, was found in 1·6% of lymph nodes sampled [Reference Brichta-Harhay43], while a second study found Salmonella prevalence in lymph nodes destined for grinding varied (0–88%) by origin of the cattle [Reference Haneklaus44]. Salmonella has also been identified in peripheral lymph nodes collected from cattle carcasses at slaughter [Reference Brichta-Harhay43–Reference Gragg45]. Interventions applied to the outside of beef carcasses would not likely affect Salmonella in lymph nodes; peripheral lymph nodes are not generally removed before grinding [Reference Brichta-Harhay43–Reference Gragg45]. Hypotheses about how Salmonella is introduced into peripheral lymph nodes include dermal abrasions or insect bites [Reference Gragg45]. Methods to decrease Salmonella in peripheral lymph nodes, such as Salmonella vaccination or pest management, might decrease contamination of ground beef [5, Reference Brichta-Harhay43, Reference Gragg45, Reference Frenzen46]. Currently, USDA-FSIS cultures randomly selected samples of beef products collected from plants through the PR/HACCP Salmonella Verification Sampling Program; if the number of positives exceeds the limit for ground beef (five in a 53 sample set) [41], USDA-FSIS takes follow-up actions, which could include additional sampling or food safety assessments, and the establishment must take corrective action or cease production [41, 47]. USDA-FSIS has proposed two changes to the Salmonella Verification Sampling Program: (1) testing additional samples of raw beef for Salmonella, such as manufacturing and bench trimmings, and other components, and (2) increasing the volume of ground beef in each sample analysed (from 25 g to 325 g) [47]. These changes might improve the detection of Salmonella and provide information about contamination of components.
Outbreaks attributed to beef, particularly ground beef, continue to occur despite current efforts. Although proper irradiation of food does not pose health risks [Reference Frenzen46] and the use of ionizing radiation to decrease pathogens in refrigerated and frozen uncooked meat has been permitted since 2000, irradiation of raw meat is still not accepted by most of the US population [Reference Frenzen46]. Most ground beef-attributed outbreaks were from food prepared at home, where the risk of improper food handling and preparation might be higher than in a restaurant or delicatessen [48]. Studies indicating that one-third of Americans fail to use safe food-handling practices to prevent cross-contamination in the kitchen [Reference Altekruse49], and that almost 20% of persons consuming ground beef say it was raw or undercooked [Reference Taylor12], suggests that knowledge and practices of consumers and food handlers could be improved through sound risk communication and behavioural science to effect positive changes in food-handling and cooking practices could help (e.g. http://www.cdc.gov/foodsafety/prevention.html, http://www.fsis.usda.gov/wps/portal/fsis/topics/food-safety-education). Consumers should continue to be vigilant about preparation of ground beef products and prevention of cross-contamination in the home. It is important to continue to reduce the contamination of meat and stronger measures are needed to prevent Salmonella contamination of beef, including additional or novel methods of pathogen reduction during processing, reducing the prevalence of Salmonella on the farm, and promoting antimicrobial stewardship. Enhancing surveillance for, investigation of, and traceback during outbreaks can decrease illnesses during an outbreak and final reporting of outbreaks to CDC can provide important scientific support of food safety interventions and policies to prevent future outbreaks. These prevention measures should also reduce the risk of foodborne illness caused by other pathogens and food vehicles.
ACKNOWLEDGEMENTS
We thank Robert V. Tauxe for early guidance in the analysis and for reviewing a draft of the manuscript, Daniel Engeljohn for early guidance in the analysis, Beth Karp for helpful comments, and others in the USDA Food Safety and Inspection Service for reviewing a draft of the manuscript. We also thank the state and local public health professionals who participated in the Salmonella surveillance and outbreak investigations that made these analyses possible.
This research received no specific grant from any funding agency, commercial or non-for-profit sectors.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
DECLARATION OF INTEREST
None.








