Introduction
The middle and lower Yangtze River floodplain is the most important wintering ground for waterbirds in eastern China, with more than one million birds coming to winter here every year, accounting for about 80% of all wintering waterbirds in eastern China (Cao et al. Reference Cao, Barter and Lei2008, Reference Cao, Zhang, Barter and Lei2010). Large geese and swans are the dominant species in the Yangtze River floodplain, of which only two species (Greylag Goose Anser anser and Bean Goose A. fabalis) have increased in abundance since the 2000s. Over the same period, four other sympatric wintering species (Swan Goose A. cygnoides, Greater White-fronted Goose A. albifrons, Lesser White-fronted Goose A. erythropus and Tundra Swan Cygnus columbianus bewickii) have declined (Cao et al. Reference Cao, Meng, Zhang, Deng, Sawa and Fox2020). Numbers of Greylag Geese overwintering in eastern China have increased from 13,000 in the early 2000s (Cao et al. Reference Cao, Barter and Lei2008) to 30,000 in 2020 (Yan et al. Reference Yan, Yi, Zhang, Batbayar, Xu, Liu and Hu2020).
Greylag Geese in East Asia mostly winter in East China and have been divided between two biogeographical sub-populations: the eastern Mongolia/Inner Mongolia-China flyway and the western/central Mongolia-China flyway. Geese that breed in the north of the Xinjiang Uygur Autonomous Region, China, and in Mongolia, through Inner Mongolia to western parts of Heilongjiang Province, China, almost all winter in the Yangtze River floodplain, with some 3% wintering in the Yellow River floodplain (Yan et al. Reference Yan, Yi, Zhang, Batbayar, Xu, Liu and Hu2020). Greylag Geese are herbivorous waterbirds that graze mostly on land, but also in shallow water, where they probe to extract plant organs from substrates (Snow and Perrins Reference Snow and Perrins1998). Greylag Geese breeding at Xianghai National Nature Reserve, Jilin Province, China, mainly fed on leaves, roots, stems, buds, fruits and seeds of aquatic and terrestrial plants while those wintering on Longgan Lake, Hubei Province, China, mainly concentrated in reedbeds, while showing increasing tendencies to feed on adjacent cropland (Pei et al. Reference Pei, Huang and Li2000, Yan et al. Reference Yan, Yi, Zhang, Batbayar, Xu, Liu and Hu2020).
Although Greylag Goose habitat use has been studied in Europe, there are few such studies in East Asia. Over the last 40 years, many goose populations in Europe have recovered from historic lows and are now more numerous than ever (Fox and Madsen Reference Fox and Madsen2017), including the Greylag Goose (Strong et al. Reference Strong, Redpath, Montras-Janer, Elmberg and Mansson2021). One hypothesised reason for the dramatic increases in European goose abundance is that geese have benefitted from the transition from feeding on natural vegetation to agricultural land. Cultivated crops, including reseeded grasslands, offer the same or better energy and nutrient composition than food in natural habitats and are generally available to geese in denser concentrations and over very much larger areas. Higher intake rates on agricultural land compared to natural habitats enable geese to meet their energy requirements more quickly than on natural habitats. Hence, croplands and artificial grasslands provide extended winter and staging carrying capacity for goose populations. Also, as capital breeders, the reproductive success of geese may be elevated by the high-quality crops consumed on the wintering grounds and spring stopover sites (Fox and Abraham Reference Fox and Abraham2017). In Europe, the shift of Greylag Geese to foraging on farmland has led to crop damage and conflict with agriculture (Strong et al. Reference Strong, Redpath, Montras-Janer, Elmberg and Mansson2021). Research to support mitigation of this conflict has been conducted through many studies on the habitat and foraging preferences of Greylag Geese (Madsen Reference Madsen1985, Patterson et al. Reference Patterson, Abduljalil and East1989, Amat et al. Reference Amat, Garciacriado and Garciaciudad1991, Fox et al. Reference Fox, Kahlert and Ettrup1998, Bakker et al. Reference Bakker, van der Wal, Esselink and Siepel1999, McKay et al. Reference McKay, Watola, Langton and Langton2006, Van Liere et al. Reference Van Liere, Van Eekeren and Loonen2009, Rosin et al. Reference Rosin, Skorka, Wylegala, Krakowski, Tobolka, Myczko and Sparks2012, Olsson et al. Reference Olsson, Gunnarsson and Elmberg2017, Kleinhenz and Koenig Reference Kleinhenz and Koenig2018, Montras-Janer et al. Reference Montras-Janer, Knape, Nilsson, Tombre, Part and Mansson2019, Ehret et al. Reference Ehret, Berthoud and Woog2020).
There have been few such studies to determine habitat use and selection of East Asian Greylag Geese. Exceptions are Yu et al. (Reference Yu, Wang, Cao, Zhang, Jia, Lee and Xu2017), who analysed the habitat use of just three Greylag Geese wintering at Poyang Lake, in Jiangxi Province, the most important waterbird wintering site in the Yangtze River floodplain, and showed that very few individuals from this population overwintered away from Yangtze River floodplain, using wetlands there for roosting and feeding. Meng et al. (Reference Meng, Li, Zhou, Qian, Wei, Han and Dai2018) tracked four Greylag Geese caught at the Tumuji wetland, in Inner Mongolia, China, where geese used marshland, grassland and lakes, but avoided croplands. At that time, Greylag Geese tagged with GPS/GSM loggers rarely ventured out onto adjacent cropland to glean post-harvest cereal and rice there, potentially due to human persecution outside the wetlands (Zhao et al. Reference Zhao, Wang, Cao and Fox2018). Yu et al. (Reference Yu, Wang, Cao, Zhang, Jia, Lee and Xu2017) already showed that Greylag Geese exploited croplands more than the other species at Poyang Lake (although still <16% of total time), which poses the questions: Are Greylag Geese from this biogeographical sub-population in the process of increasingly utilising farmland? Does this trend, together with greater use of protected areas throughout the annual cycle contribute to its better conservation status compared to the sympatric species with which it overwinters? We attempted to answer these questions and potentially better understand the reasons for the increase in Greylag Geese, by following individuals tagged with GPS loggers to determine habitat use and the degree to which tagged birds used protected areas throughout the annual cycle.
In this investigation, we deployed tracking devices on Greylag Geese breeding in the Dauria region and wintering in the Yangtze River floodplain. Among Greylag Goose habitat preference studies in Denmark (Madsen Reference Madsen1985), India (Middleton Reference Middleton1992), England (McKay et al. Reference McKay, Watola, Langton and Langton2006), Poland (Rosin et al. Reference Rosin, Skorka, Wylegala, Krakowski, Tobolka, Myczko and Sparks2012) and China (Meng et al. Reference Meng, Li, Zhou, Qian, Wei, Han and Dai2018), important explanatory variables included land cover type, distance to water surface, and distance to human settlements. For this reason, we chose four key parameters to model Greylag Goose habitat selection, including land cover type (each class is treated as a separate variable), the shortest distance to lakes/wetlands, roads (as a proxy for human access and disturbance) and protected areas. We also attempted to assess the effectiveness of the currently designated areas to protect the species at each stage of the life cycle.
Methods
Satellite tracking and identifying stopover sites
We captured and marked 20 Greylag Geese with neck collar-mounted GPS/GSM transmitters at three sites, two on the winter quarters and one on the breeding area. Five birds were caught at Poyang Lake (29.00°N, 116.40°E) in December 2014 and 10 birds at Anhui Lakes (30.88°N, 117.70°E, Anhui Province, China) in October/November 2016, both on the wintering grounds. Five birds were also caught at Buir Lake (47.70°N, 117.58°E, eastern Mongolia) in July 2017 during the breeding season. All fitted devices constituted <3% of an individual’s total body mass (Millspaugh and Marzluff Reference Millspaugh, Marzluff, Millspaugh and Marzluff2001, Lameris and Kleyheeg Reference Lameris and Kleyheeg2017, Bodey et al. Reference Bodey, Cleasby, Bell, Parr, Schultz, Votier and Bearhop2018). We preselected the devices to record positions at hourly intervals.
For each individual, we defined spring migration as starting from the last position received from the overwintering area (Yangtze River floodplain) and terminating with the first position of a series of positions received from the summering grounds (Mongolian Plateau); autumn migration, summering, and wintering periods followed similar definitions (Li et al. Reference Li, Wang, Fang, Batbayar, Natsagdorj, Davaasuren and Damba2020a). We applied the methods of Wang et al. (Reference Wang, Cao, Batbayar and Fox2018) to segment movement tracks, identifying flight legs between successive stopover sites, which were defined as locations where a bird did not move >30 km in a 48-h period (Kölzsch et al. Reference Kölzsch, Müskens, Kruckenberg, Glazov, Weinzierl, Nolet and Wikelski2016, Li et al. Reference Li, Wang, Fang, Batbayar, Natsagdorj, Davaasuren and Damba2020a). Using this approach, we obtained the stages during the summering/wintering period and spring/autumn migration of each track of each individual to describe the habitats that these birds used for feeding and resting. Since some individuals were tracked for more than one year, to avoid pseudo-replication, the tracks in each of the four seasons were selected only once from each individual (Table S1 in the online supplementary material).
Conservation and land-use status of habitats
We attempted to assess the effectiveness of the current extent of designated national-level nature reserves of China, Mongolia, and Russia for the protection of habitats used by tagged Greylag Geese. We downloaded the boundary information from the National Nature Reserves of China (NNRs; accessed at http://www.resdc.cn/data.aspx?DATAID=272), Database of Mongolian Protected Areas (Ministry of Nature, Environment and Tourism, Mongolia; accessed at www.mpa.gov.mn/gis) and World Database of Protected Areas for Russia (WDPA 2018; accessed at protectedplanet.net).
To characterise the land cover habitat types exploited by tagged geese at all stages of the annual cycle, we used the “FROM-GLC 2017” land cover dataset (resolution 30 m × 30 m) created by the Department of Earth System Science, Tsinghua University (Gong et al. Reference Gong, Liu, Zhang, Li, Wang, Huang and Clinton2019). These classify land cover according to 10 habitat types: cropland, forest, grassland, shrubland, wetland, water, tundra, impervious surface, bare land and snow/ice with an estimated 72% accuracy (Gong et al. Reference Gong, Wang, Yu, Zhao, Zhao, Liang and Niu2013, 2019).
In order to exclude positions recorded by the GPS units when the geese were flying (and therefore bore no relationship to the habitats within which they were recorded), we only retained the GPS locations where the velocity between adjacent points was less than 1 km h–1 (Zhang et al. Reference Zhang, Xie, Li, Batbayar, Deng, Damba and Meng2020). In order to reduce the influence of varying time intervals between sequential positions recorded by the GPS unit caused by voltage changes, we diluted the GPS data down to one point per hour (Signer et al. Reference Signer, Fieberg and Avgar2019). On this basis, we finally generated 48,376 GPS fixes of which 94.4% were positions with 1-h intervals from the next. We then overlaid these positions on GIS layers containing nature reserves boundaries and land cover images using QGIS 3.20, to identify which points were located within nature reserves and to assign each to specific land-use types. GPS fixes were assigned to day or night based on local sunrise and sunset times calculated by “suncalc” package (Thieurmel and Elmarhraoui Reference Thieurmel and Elmarhraoui2019) to compare differences between day and night. We defined day as the time between one hour before sunrise and one hour after sunset, and defined the rest as night.
Habitat selection modelling
We used generalized linear mixed models (GLMMs) with a binomial error structure to evaluate habitat resource selection in four seasons separately (Zhang et al. Reference Zhang, Xie, Li, Batbayar, Deng, Damba and Meng2020). Use/availability were entered into models as the response variable, environment variables (land cover type, the shortest distance to lakes/wetlands, roads and protected areas) as explanatory variables and transmitter ID and year as random variables (Meng et al. Reference Meng, Wang, Batbayar, Natsagdorj, Davaasuren, Damba and Cao2020).
Use data were generated using the methods outlined above and compared to availability data (i.e. pseudo-absence data) which were generated for each season using the following method. We created 100% minimum convex polygons (MCPs) based on each set of positions for tagged geese and extended these outwards by the average maximum hourly displacement for all individuals in all directions around the MCPs (summering 13.8 km, wintering 15.7 km, spring/autumn migration 12.2/12.1 km) in order to represent the area potentially available to each of the staging birds. We then randomly selected locations from the extended MCP for each site as availability data, generating 20 pseudo-absence points for each positional fix to gain stable and unbiased parameter estimates (Northrup et al. Reference Northrup, Hooten, Anderson and Wittemyer2013).
We extracted land cover type from “FROM-GLC 2017” land cover dataset, and excluded rare land cover types in each season (<5% of total land use by either use or availability data points) to escape model convergence problems likely below such levels (Altman et al. Reference Altman, Gill and McDonald2004). We calculated the shortest distance of each use or availability data points to lakes/wetland (Global Lakes and Wetlands Database created by WWF and the Center for Environmental Systems Research, University of Kassel, Germany), roads (downloaded from https://www.worldclim.org/) and national-level protected areas, respectively. Finally, we rescaled variables using the “scale” function in R (Becker et al. Reference Becker, Chambers and Wilks1988) to estimate the effect size of explanatory variables, and calculated Pearson correlation between variables to make sure there is no correlation greater than 0.75 and hence no need to delete variables.
We used the “dredge” function in “MuMIn” package (Bartoń Reference Bartoń2019) in R to develop our resource selection model using model weights derived from AICc criteria. The cross-prediction accuracy of our resource selection model was tested by estimating the area under the receiver operating characteristic (ROC) curve (AUC). AUC values can range between zero and one, where one indicates perfect discrimination, with values above 0.7 being generally accepted as indicating reasonable predictions (Hosmer Jr et al. Reference Hosmer, Lemeshow and Sturdivant2013).
Results
Overall, we obtained entire seasonal tracks from 10 tracked Greylag Geese (Table S1). These comprised six tracks in summer (n = 2 in 2015; n = 3 in 2017; n = 1 in 2018), eight in winter (n = 2 in 2015; n = 2 in 2016, tracked in the early winter; n = 4 in 2017), eight in spring (n = 2 in 2016; n = 5 in 2017; n = 1 in 2018) and eight in autumn (N = 2 in 2015; n = 6 in 2017, one bird completed migration without stopovers).
Distribution and conservation status of habitats
In summer, Greylag Geese used the Mongolian Grassland Biome (including CMR-Dauria International Protected Area, Huihe NNR, and Wulagai Wetland, see Table 1 and Figure 1) as their summering grounds (with an average duration of 186 ± 8 days; Ng = 22,371, number of GPS fixes) and 55% of the GPS locations fell within the national-level protected areas of three countries. In winter, geese used the Yangtze River floodplain (including Poyang Lake, Longgan Lake and Anqing Lakes, China) as their wintering grounds (104 ± 22 days; Ng = 15,688), when 43% of GPS locations fell within the NNRs. During spring migration, geese mainly used Bohai Bay (including Yellow River Estuary and Nandagang Reservoir, China; 26 ± 9 days) and the Mongolian Grassland Biome (including Wulagai Wetland and XilaMulun River, China; 11 ± 5 days) as stopover sites, when 48% of GPS locations (Ng = 4,425) fell within the NNRs. During autumn migration, geese mainly used Mongolian Grassland Biome (including Wulagai Wetland and XilaMulun River, China; 35 ± 1 days) and Bohai Bay (including Beidagang Reservoir, Nandagang Reservoir and Yellow River Estuary, China; 40 ± 14 days) as stopover sites and 45% of GPS locations (Ng = 5,892) fell within the NNRs.
Table 1. Habitat location of Greylag Geese Anser anser tracked using satellite transmitters. Only staging sites where the estimated duration of stay was at least two days are shown. Duration was shown by mean ± standard deviation; n, number of tracked individuals, Ng, number of GPS fixes; Protected%, percentage of Ng located in national-level protected areas.
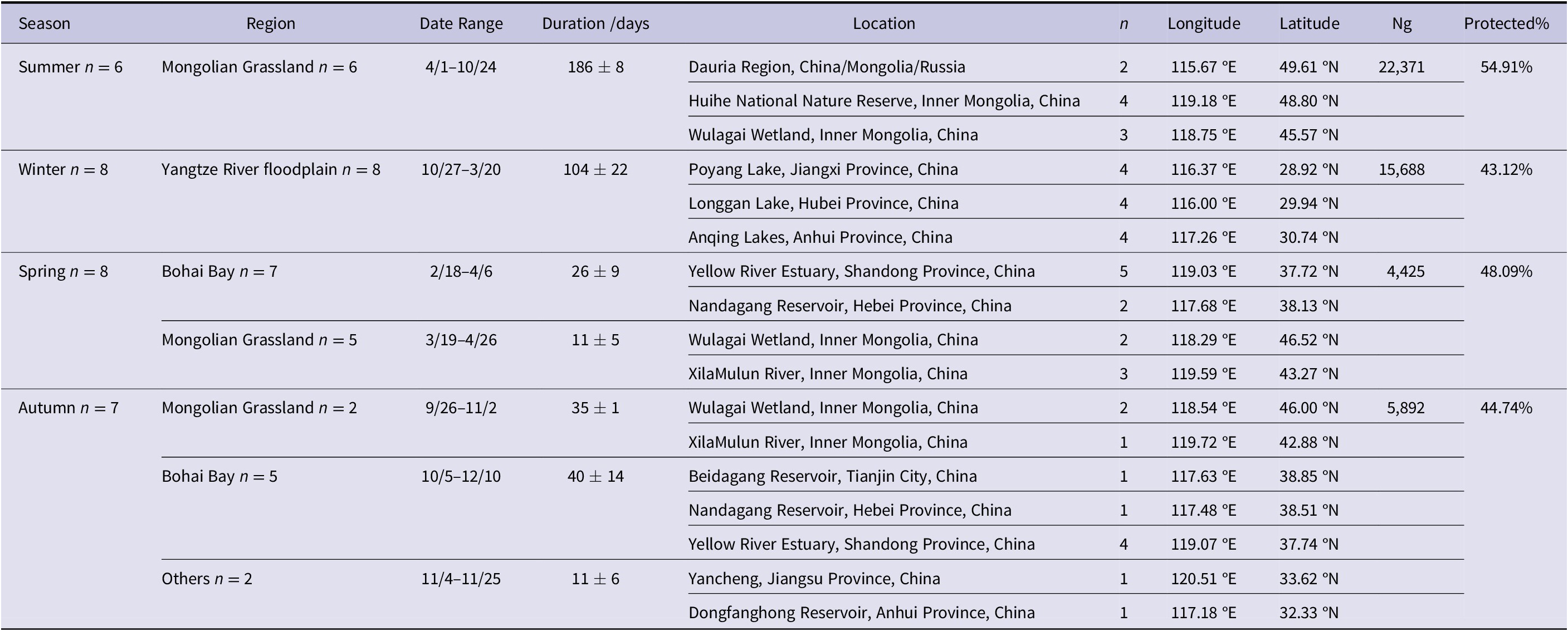

Figure 1. GPS fixes of Greylag Geese Anser anser in summering/wintering areas and spring/autumn migration stopover sites. The areas enclosed by the red line represents the summer and winter home ranges which were the 100% maximum activity areas for these seasons calculated based on all GPS fixes, shown expanded in the maps depicted far left.
Habitat use and selection in different periods
We overlaid the tracking data from the Greylag Geese in the different seasons upon the land cover dataset to obtain the habitat use for each of the birds. The combined results showed that Greylag Geese used 14% bare land, 13% cropland and 3% forest in summer, as well as 20% and 7% forest in spring and autumn respectively. Such habitat use is not consistent with the results of previous studies or from our own observations, since Greylag Geese usually avoided areas with tree cover or bare substrates, instead foraging in croplands, grasslands, and wetlands, but mainly on grass in summer. Hence, we checked the habitat use results based on satellite images and field surveys. In summer, GPS fixes identified from bare land in the Dauria Region, Huihe NNR and Wulagai Wetland, were actually distributed within areas shown as dry lakes on the imagery (Ng = 3,261, Figure S1), since ephemeral dry lakes were defined as bare land in the “FROM-GLC 2017” dataset (Gong et al. Reference Gong, Wang, Yu, Zhao, Zhao, Liang and Niu2013), so Greylag Geese potentially used waterbodies or bare land at these sites, depending on local hydrology, but were assigned to dry land. GPS fixes identified as cropland and forest in Huihe NNR, were actually located in single species stands of common reed Phragmites australis and bulrush Typha orientalis, so we changed the land cover type for these fixes to wetland (Ng = 3,670, Figure S2). In spring and autumn, GPS fixes identified as forest in Nandagang and Yangcheng Reservoir, Hebei Province, also were obviously located in reedbeds, so the land cover type of these fixes were also changed to wetland (Spring: Ng = 869, Autumn: Ng = 392, Figure S3).
Finally, after modifying the incorrectly assigned land cover types, the habitat types used by the Greylag Geese were predominantly natural ecosystems in summer (52% grassland and 30% wetland/water body), but a relatively large proportion of cropland in other seasons (46% cropland in winter, 17% and 20% in spring and autumn). Habitats used by geese were similar during day and night in summer, winter, and autumn, but differed in spring (see Figure 2), when they used wetland/water body more by day (61% by day and 53% at night) and cropland at night (10% by day and 28% at night).

Figure 2. Percentage habitat use of Greylag Geese by day (A) and night (B) during four seasons respectively, based on GPS fixes overlaid on land-cover maps of the world. N: number of instrumented individuals generating the data; Ng: number of GPS fixes.
Comparing GLMM results, the models with best fit for predicting habitats were based on similar parameters across all four seasons (AUCsummer = 0.86, AUCwinter = 0.72, AUCspring = 0.85, AUCautumn = 0.75). These included the shortest distance to roads, lakes/wetlands and national-level protected areas, as well as five common habitat types (wetland, water, grassland, cropland, and bare land), although water and cropland did not contribute in summer and grassland and bare land did not contribute in winter, when lakes might be expected to be full of water. All these parameters were significant (P < 0.01; Table 2). Among habitat types, Greylag Geese tended to select wetlands (βsummer = 4.87, βwinter = 4.13, βspring = 4.64, βautumn = 4.39) in all seasons, and strongly select cropland (βwinter = 2.50, βspring = 1.08, βspring = 1.56) in winter, spring and autumn. Geese tended to strongly select areas close to lakes/wetlands (βsummer = –1.36, βwinter = –0.39, βspring = –1.00, βautumn = –0.83) in all four seasons, and areas close to roads (βsummer = –0.61, βwinter = –0.40) in summer and winter (see Table S2 for full model result).
Table 2. The result of habitat selection ratios for Greylag Geese tracked using satellite transmitters during four seasons.

Note: ‘***’ indicates P-value <0.001; ‘**’ indicates P-value <0.01. ‘–’ indicates the land cover type did not participate in the model.
Discussion
Selection for wetlands in all seasons
We used freely available Earth remote sensing data in combination with knowledge of precise movements of tagged Greylag Geese for the first time in this biogeographical sub-population to assess habitat selection throughout their entire annual cycle. According to habitat selection models, we found that Greylag Geese tended to choose wetlands and areas close to lakes/wetlands as their habitat in all seasons. In summer, Greylag Geese also tended to choose areas close to roads, which may be caused by the road distribution network near wetlands in Dauria Region and Huihe NNR. In winter, Greylag Geese tended to choose wetlands, water bodies and cropland, very similar to the same species wintering in England, where numbers increased with the farmland area and proximity to water (McKay et al. Reference McKay, Watola, Langton and Langton2006). At the same time, the results of habitat use also confirmed that Greylag Geese used more than 50% wetlands/water bodies during winter and migration seasons, indicating that wetlands are still very important for Greylag Geese, not least for daytime loafing, drinking, and night-time roosts.
Cropland use during the non-breeding seasons
Greylag Geese mainly used natural ecosystems in summer, in contrast to the enhanced use of croplands in association with wetlands/water bodies, which predominated during winter and migration periods. These patterns are similar to patterns shown by Greylag Geese breeding in Europe. During the moulting period, Greylag Geese foraged mainly on short grasslands (0.5–10 cm) in Sweden (Strong et al. Reference Strong, Redpath, Montras-Janer, Elmberg and Mansson2021) and foraged in the fields and meadows around lakes in south Germany to which they resort to gain safety from predators (Kleinhenz and Koenig Reference Kleinhenz and Koenig2018). In winter, Greylag Geese also preferred eating grain and corn in fields by day and roost on open water bodies by night in south Germany (Kleinhenz and Koenig Reference Kleinhenz and Koenig2018) and in the past have also been widely distributed in marshes and farmed fields around Lake Ichkeul in the Ichkeul National Park, Tunisia (Hamdi et al. Reference Hamdi, Charfi and Moali2008). During the spring migration of 2007–2013, Greylag Geese used arable lands frequently in the Biebrza Basin, north-eastern Poland (Polakowski and Kasprzykowski Reference Polakowski and Kasprzykowski2016). During autumn migration, Greylag Geese foraged mainly on wheat and corn stubbles in Denmark and west Poland (Madsen Reference Madsen1985, Rosin et al. Reference Rosin, Skorka, Wylegala, Krakowski, Tobolka, Myczko and Sparks2012), but mostly on Scirpus tubers and Spartina rhizomes on the island of Schiermonnikoog, Netherlands (Bakker et al. Reference Bakker, van der Wal, Esselink and Siepel1999). It seems relatively rarely that in Europe, Greylag Geese still resort to natural wetland communities to feed, as in the Donana National Park, where they do so to feed on below-ground Scirpus tubers to the present day (Amat et al. Reference Amat, Garciacriado and Garciaciudad1991). In conclusion, it begins to look as if the habitat use of Greylag Geese in East Asia is increasingly resembling that in Europe, especially in winter, spending large proportions of time foraging in artificial agricultural ecosystems. This trait could also have contributed to an increase in their overall fitness and hence to population change.
Outside the breeding season, however, Greylag Geese in East China showed relatively equal probability for feeding on cropland by day and night, especially during autumn and winter, but tended to feed more on fields at night in spring. This may relate to daytime agricultural activities, for instance in relation to the large numbers of free-range poultry in China, which are often tended by farm workers and which displace Greylag Geese foraging in fields (Zhao et al. Reference Zhao, Wang, Cao and Fox2018). By feeding at night, geese avoid humans and the potential risk of persecution and hunting, as well as aggressive interactions with domestic poultry. Greylag Geese moulting in Denmark also fed at night to reduce the risk of predation there (Kahlert et al. Reference Kahlert, Fox and Ettrup1996). Studies of Eastern Tundra Bean Geese A. fabalis serrirostris at Shengjin Lake, China, also suggested a shift amongst this species from daytime to night-time feeding in mid-winter, in that case also potentially to avoid interspecific competition with Greater White-fronted Geese (Zhao et al. Reference Zhao, Cao, Klaassen, Zhang and Fox2015). Much of the cropland selected by Greylag Geese in China during winter was conspicuously located adjacent to wetlands or lakes (Figure S4), as is the case for Greylag Geese in southern Sweden (Nilsson and Persson Reference Nilsson and Persson1992) and is consistent with minimising energetic costs of commuting between feeding areas and safe night-time roosts.
It seems highly likely that the energy and nutrient intake rates of Greylag Geese are enhanced by foraging in croplands relative to feeding on traditional natural habitats, all other conditions being similar. Compared with natural food, crops are mostly higher in energy, lower in fibre and ash, and, being widespread and grown in monocultures, easier to obtain (Fox and Abraham Reference Fox and Abraham2017). During spring, Greater Snow Geese Chen caerulescens atlantica staging in southern Quebec, Canada, derive 3.5–4.5 times the metabolizable energy from feeding on spilled grain in stubbles than on wild Scirpus americanus and Spartina alterniflora in their traditional intertidal marsh, due to the greater grubbing, extraction and handling time needed in marshes (Béchet et al. Reference Béchet, Giroux and Gauthier2004). In addition, the time needed for Brant Goose Branta bernicla bernicla to fulfil nitrogen needs was far less on winter wheat (3.7 h·day–1) compared to saltmarsh (considered the most natural habitat, 11.25 h·day–1; Hassall and Lane Reference Hassall and Lane2005). It will be instructive to investigate the relative cost/benefit trade-offs between Greylag Geese foraging on natural and artificial food resources in Yangtze River floodplain wetlands where such possibilities exist side-by-side. This could be achieved by comparing the energetic and behavioural advantages of feeding on croplands versus natural wetlands simultaneously while these two feeding traits are still evident in this population.
The fact that Greylag Geese in East China used more cropland on their wintering and staging sites may be related to the increasing use of mechanical harvesting in China, which has increased the amount of spilled grain available as food as well as reducing levels of human disturbance. As recently as the 2000s, workers planted, tended, and harvested crops by hand in the fields, which resulted in high intensity disturbance over protracted periods, that in turn caused considerable interruptions to foraging by all waterbirds, including geese, foraging in the fields (Zhao et al. Reference Zhao, Wang, Cao and Fox2018). However, this pattern of agricultural work changed rapidly after the Ministry of Agriculture of the PRC issued the “Opinions on Accelerating the Mechanization of Rice Production” in 2011. In 2000, tractor density per 100 km2 of arable land in the Netherlands was 32 times that in China (see: http://data.worldbank.org/indicator/AG.LND.TRAC.ZS). Before 2011, only 20% of rice planting and 25% of corn harvesting were mechanized in China; by 2015, this had risen to 45% of rice planting and 80% of harvesting. By 2021, levels of wheat, rice and corn machine harvesting in China had reached 97%, 94% and 78% respectively (Data source: www.gov.cn, the website of the Central People’s Government of the PRC). The result is that radically fewer workers are active in cropland fields over a far shorter time period, with far less human interference to birds foraging in the fields. The cropland types in the middle and lower Yellow River and Yangtze River floodplain account for more than 30% of those for all of China according to the statistics of land cover atlas of China in 2015 (Wu et al. Reference Wu, Qian, Zeng, Zhang, Yan, Wang and Li2017). It has been estimated that if the rice loss due to unharvested grain during harvest were to be reduced from 3.02% to 2.76%, then an addition 540,000 tons of rice could be added to the harvest in China, showing the potential food available to stubble foraging birds (Huang et al. Reference Huang, Yao, Wu and Zhu2018). This indicates that there are very large quantities of waste grain available in the Yellow River and the Yangtze River floodplain agricultural areas, which can potentially provide ample food sources for geese. The wintering and staging sites of Greylag Geese in East China lay in these two areas, which have both likely benefitted most from the spread of mechanical harvesting. Studies have shown Greylag Geese coexist with other bird species while foraging (Randler Reference Randler2004), thus for example, they can feed on spilled grain in the environment shared with large numbers of free-range poultry. By weighing the energy costs of disturbance by human and poultry, the benefits of foraging on cropland may outweigh the benefits of foraging in wetlands, which may explain why the Greylag Goose in East China have begun to use cropland more than was apparently the case in the past. In that respect it is interesting to compare the contemporary use of cropland by Greylag Geese in winter now from this study (46%) with that of 15% (Yu et al, 2017). This increase could be partly explained by the use of different land use cover datasets (which used much earlier and more spatially restricted land use cover data) and small sample size, but at least these data contribute to a time series, which could in the future potentially track long term changes in the species’ use of agricultural land over time.
Another possible reason for geese increasingly using agricultural fields is the simultaneous degradation of natural wetlands in the Yangtze River Floodplain. For example, at Poyang Lake, submerged plants have degraded seriously in the last decade (Li et al. Reference Li, Zhong, Shao, Yan, Jin, Shan and Li2020b). The loss of the submerged plant biomass has resulted in cranes shifting from foraging in natural wetlands to agricultural fields (Hou et al. Reference Hou, Liu, Fraser, Li, Zhao, Lan and Jin2020, Reference Hou, Li, Wang, Wang, Zhan, Dai and Lu2021a). In addition, the increasing loss of submerged, tuber-producing Vallisneria has resulted in the decline in Swan Goose abundance at Shengjin Lake (Zhang et al. Reference Zhang, Cao, Barter, Fox, Zhao, Meng and Shi2011). This widespread loss and degradation of the feeding resource in natural wetlands is likely to influence the choice of foraging habitat by Greylag Geese, because submerged plants are important food items for this species as well.
Elevated use of national-level protected area in all seasons
The relatively high level of conservation site protection in areas used by Greylag Geese in all seasons (55% in summer, 43% in winter, 48% and 45% in spring/autumn migration) is encouraging and is likely also a potential contributory factor that contributes to demographic effects that have supported recent increases in abundance. In addition, very recent sympathetic management and restoration of natural conditions at national nature reserves have created a much-improved environment for waterbirds, including geese. For instance, the major ecological restoration policy and programme to return farmland to wetlands at Longgan Lake NNR (wintering grounds for Greylag Geese) and Yellow River Delta NNR (an important staging site for many Greylag Geese; some of which also remain in summer) has banned net fishing operations at these sites, specifically to protect migratory birds and to restore a greater extent of suitable waterbird habitat (Yan et al. Reference Yan, Yi, Zhang, Batbayar, Xu, Liu and Hu2020). The measures in Yellow River Delta included returning 4,800 ha of cropland and fish netting shallow water to undisturbed wetland and coastal marsh, restoring 200 ha of coastal wetlands in 2020 (Data source: www.gov.cn). This major programme of habitat restoration offers a major opportunity for us to observe, using continued future tracking of individual geese, how waterbirds respond to sympathetic management of their environment, for example, by comparing their previous behaviour and habitat selection and use in the area in recent years, with those in future post-restoration. Furthermore, more than 80,000 ha and 60,000 ha of paddy fields, respectively, around the two reserves are increasingly the main attraction to large numbers of migratory birds using these areas (Yan et al. Reference Yan, Yi, Zhang, Batbayar, Xu, Liu and Hu2020, Hou et al. Reference Hou, Li, Xu and Wang2021b).
Juvenile Greylag Geese, the relatively more naïve and adventurous element of the population, which normally has lower survival rates, may experience reduced mortality as a result of increasing their use of farmland and protected areas. Based on four surveys undertaken in the middle and lower Yangtze River floodplain between 2016 and 2019, the mean age ratio (i.e. the proportion of juveniles in relation to total numbers) among Greylag Geese was the highest among the five large goose species, reaching 0.19 (n = 25,339; Wang Reference Wang2021). However, even when such data on annual reproductive success and survival are available, establishing the causative environmental factors responsible for population change remain a major challenge. For this reason, we need to undertake further telemetry studies, ideally, tracking individuals from separate flocks of Greylag Geese that forage only on wetlands and exclusively on farmland, to monitor the differences in nutritional and energetic intake rates and changes in their respective reproductive success and/or survival rates. This presupposes that individual Greylag Geese show such discrete and consistent habitat preferences, but the results would be interesting if such studies proved to be possible and would offer some support for confirming the fitness consequences of habitat selection in this flyway of Greylag Geese.
Conclusions
Although we urge prudence in concluding too much from so few marked birds, in general, we speculate that we have support for the fact that two factors may have contributed to recent population increases in Greylag Geese in East Asia: (1) Greylag Geese have increasingly shifted to feeding on cropland in non-breeding seasons (46% in winter and 20% during migration), which might have improved energy intake efficiency. (2) the level of protection for sites used by tagged Greylag Geese in all seasons is high (55%, 43%, 48% and 45% in summer, winter, spring, and autumn respectively), which implies some reasonable level of protection of favoured habitat against loss compared with unprotected sites, as well as reduced disturbance within these sites. As the population continues to increase, we predict that more and more Greylag Geese will shift to feeding on croplands and benefit from the abundant high-quality artificial food to be found there. However, because of the timing of Greylag Goose migration, more geese are increasingly likely to forage on unharvested and new sown fields. This development will inevitably also bring a risk of longer-term conflicts with agriculture, as has happened in many European countries (Montras-Janer et al. Reference Montras-Janer, Knape, Nilsson, Tombre, Part and Mansson2019). For this reason, we consider it important to continue to monitor the situation and to pay more and longer attention to the behaviour and habitat use of geese in China to ensure their most effective conservation management and that of the wetlands upon which they have been dependent, in the future.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/S095927092200017X.
Acknowledgements
We gratefully acknowledge the contribution of Xueqin Deng and Lei Fang for their help on field works and data analysis, and the fieldwork teams in China and Mongolia for their contributions. Our study was supported by the National Natural Science Foundation of China (Grant No. 32100373), the Scientific Instrument Developing Project of the Chinese Academy of Sciences (Grant No. YJKYYQ20180050) and China Biodiversity Observation Networks (Sino BON).






