Chronic malnutrition is highly prevalent in sub-Saharan Africa, especially among poor rural households( Reference Black, Allen and Bhutta 1 ), and it is mainly caused by morbidity and inadequate dietary intake( 2 ). Infants and young children are most affected by the physical and mental deficits occurring due to chronic malnutrition. These deficits are carried over into the school-age period, where they retard cognitive function, educability and future productivity( Reference Black, Allen and Bhutta 1 ). Most interventions at the household and community levels are, however, preferentially targeted outside the first 1000 d of life( Reference Black, Allen and Bhutta 1 , Reference Bundy, Burbano and Grosh 3 ). ‘The school’ may serve as a platform for targeted interventions, such as school feeding programmes (SFP), to contribute to the fulfilment of the nutritional needs of children outside the first 1000 d of life. However, in settings where school enrolment and attendance are low, targeting interventions at schoolchildren may still be problematic. In Africa and other developing continents, SFP have therefore been instituted primarily as food-for-education programmes in resource-poor settings not only to improve school enrolment and attendance but also as means to improve nutritional status through improved energy and nutrient intakes( Reference Adelman, Gilligan and Lehrer 4 ).
Following the formulation of the UN Millennium Development Goals, SFP received renewed interest for their potential contribution to the achievement of Millennium Development Goals 1 and 2. In line with the recommendations of the UN Hunger Task Force, there has been a shift in the paradigm of SFP towards linking local food production to consumption at schools (home-grown school feeding) with the aims of creating and improving access to market for poor rural farmers, stimulating local food production and also improving the local economy of beneficiary communities( 5 ). The shift in paradigm received support from African Governments through the Comprehensive Africa Agricultural Development Programme of the New Partnership for Africa's Development, thereby putting SFP on the political agenda of Africa( 6 ). Since 2005, the Government of Ghana has piloted and up-scaled the Ghana School Feeding Programme through which schoolchildren are provided one nutritious meal per school day to encourage educational participation (enrolment, attendance and retention) and also improve nutrient intake and nutritional status( 7 ).
However, comprehensive reviews of empirical research( Reference Bundy, Burbano and Grosh 3 , Reference Adelman, Gilligan and Lehrer 4 , Reference Kristjansson, Robinson and Petticrew 8 ) and programme evaluation reports( Reference Buttenheim, Alderman and Friedman 9 – Reference Kazianga, de Walque and Alderman 11 ) have shown that although SFP have had positive impacts on educational participation, their impacts on nutritional outcomes have been rather unclear( Reference Bundy, Burbano and Grosh 3 , Reference Adelman, Gilligan and Lehrer 4 , Reference Kristjansson, Robinson and Petticrew 8 – Reference Kazianga, de Walque and Alderman 11 ) and partly blamed substitution effect of school feeding on home consumption for the lack of effect. Moreover, in the Lancet series on Maternal and Child Undernutrition, SFP targeting children aged >2 years have been described as interventions that are unlikely to improve nutritional status( Reference Bryce, Coitinho and Darnton-Hill 12 ) because such interventions are outside the window of opportunity for improvement in nutritional outcomes, particularly stunting.
Without evidence of a positive impact on nutritional outcomes, it is unlikely for SFP to improve cognition and academic performance despite the demonstrable improvements in educational participation. Therefore, in the present study, we aimed to assess the nutrient intake adequacy and Fe and nutritional status of schoolchildren participating in a government-supported SFP in northern Ghana relative to non-participating children.
Subjects and methods
Study design
This was a cross-sectional study involving the quantitative measurement of energy and nutrient intakes and Fe and nutritional status of children in school feeding and non-school feeding schools. At the inception of the government-supported pilot SFP in northern Ghana in October 2005, baseline usual nutrient intakes and Fe and nutritional status of schoolchildren in the study area were not measured. Therefore, in the present study, only children in schools participating in a SFP were compared with their neighbouring non-school feeding counterparts at a point in time. At the time of the study, the government-supported SFP had been operational in the study area for about 3 years and all children in primary school in both beneficiary schools received lunch at school, but not all participated in the present study.
Data collection for the survey was conducted over a period of 1 month (1st week of November 2008 to 2nd week of December 2008). Ethical clearance was given by the Institutional Review Board of Noguchi Memorial Institute for Medical Research, University of Ghana (NMIMR-IRB 022/08-09). Permission was also sought from the District Administration, District Education Office, head teachers and local authorities in each community. After information sessions, parents/caregivers who volunteered to participate in the study gave written informed consent.
Study area
The study was conducted in four of the 132 primary schools in Tolon-Kumbungu District of Northern Region, Ghana. The four rural primary schools (from four communities) were approximately 50 km away from the main city in the region, Tamale, and within 5 km radius from each other. Of these schools, two (Tibung and Kpalgung primary) were the pilot schools for the government-supported SFP in Tolon-Kumbungu District, which started in October 2005 and was still running at the time of the study. The other two schools (Wayamba and Jegbo primary), which qualified to benefit from SFP but were not yet enrolled, were selected as control schools based on their similarity with the pilot schools with respect to the following characteristics: number of children enrolled in school; school infrastructure; size of community; absence of market infrastructure; water and sanitation facilities; proximity to each other. The study area is within the Guinea Savanna vegetation zone, having a typical unimodal rainy season (April–September) and one dry season (December–March) characterised by relatively high temperatures (35–40°C). People living in this area are mostly subsistence farmers( 13 ). Malaria is hyperendemic in this area( Reference Ehrhardt, Burchard and Mantel 14 ) and is the main cause of morbidity among children( 13 ). Malaria transmission peaks towards the end of the rainy season (October and November)( Reference Owusu-Agyei, Koram and Baird 15 ).
Subjects and sampling
Subjects
Children (aged 5–13 years) in classes 1–3 from the four schools in Tolon-Kumbungu District were included if they were enrolled in school for at least one academic year at the time of the study. Mothers or alternate caregivers were interviewed to obtain data on the dietary intake of children because they prepared and served meals in the households.
Sample size and sampling procedure
Due to paucity of literature on the usual nutrient intake of schoolchildren in the study area, anaemia prevalence (proxy for Fe status) among schoolchildren was used for the determination of sample size. Based on an assumed anaemia prevalence of 50 % among non-school feeding participants, a sample size of about 180 children per group was required to estimate a 15 % point difference in anaemia prevalence between the school feeding and non-school feeding groups with 95 % confidence (one-sided) and a power of 90 %. Taking into account 10 % attrition, sample size was rounded to 200 per group.
A total of 383 schoolchildren were recruited for the study: 196 from the school feeding group and 187 from the non-school feeding group. In each of the four schools, children were randomly selected from a sampling frame of pupils in lower primary (classes 1–3). The sampling frame was constructed separately for each school by pooling together the registers of lower primary. If two or more children were selected from one household, one of them was randomly selected by lottery to participate in the study.
Data collection and measurements
Household questionnaire
A semi-structured survey instrument was used to collect information on the sociodemographic characteristics of children and their households. Parents/caregivers were asked to indicate whether their child was ill during the 2 weeks preceding the survey. The instrument also included the standardised and validated( Reference Deitchler, Ballard and Swindale 16 ) Food and Nutrition Technical Assistance Household Hunger Scale (HHS). The HHS is a three items-by-three frequencies of occurrence scale and was used for the assessment of the food supply situation of participating households( Reference Ballard, Coates and Swindale 17 ). The survey instrument was translated into the local language (Dagbani) and pretested by trained research assistants before being used in the survey. The standard reference period of 30 d was used for the HHS assessment( Reference Ballard, Coates and Swindale 17 ).
24 h recall method
Quantitative 24 h recalls (24 hR), repeated in 20 % subsample, were collected by six trained research assistants (first degree nutrition graduates), who spoke the local language and had knowledge of the study area. A minimum duration of 2 d was allowed between repeated recalls to avoid dependency of intake on two consecutive days, especially caused by the consumption of leftover foods( Reference RS and EL 18 ). Weekend days were excluded. Days of the week and interviewers were randomly allocated to children to account for differences between days and interviewers, and interviewers were not allowed to interview the same household twice. All 24 hR were completed within the same post-harvest season, a period of minimum food scarcity.
A standard multiple-pass procedure was used for all 24 hR( Reference RS and EL 18 ). First, mothers/caregivers were asked to provide details on all foods and beverages that their child had consumed during the preceding 24 h (wake up-to-wake up) including anything consumed outside home. After probing for likely forgotten foods with the help of the index child( Reference Gewa, Murphy and Neumann 19 ), they were asked to give a detailed description of foods and beverages consumed, including ingredients and cooking methods for mixed dishes and place and time of consumption. The amount of each food and beverage and ingredients of mixed dishes was weighed or, when not available, estimated in household measures or their monetary equivalent. The weight of foods and ingredients of mixed dishes was measured using a digital kitchen scale (Soehnle Plateau, model 65 086), precise to 2 g with a maximum capacity of 10 kg. Factors for converting household measures and monetary values into weight were determined afterwards. The total volume of all foods and mixed dishes cooked, volume consumed by child and leftover from child's food were determined to derive the proportion of total prepared food consumed by the child. For SFP participants, the 24 hR did not include detailed recall of lunch served at school under SFP. Rather, the weighed food record (done at school) was used to measure the quantity of lunch consumed (see the ‘Weighed food record’ section).
Communal eating is a common practice in this area; therefore the number of children who shared meals with the index child was obtained and used as a divisor to obtain an estimated quantity of food consumed by the index child. In such situations, equal sharing of food was assumed. The weight of the various ingredients consumed by the child was obtained by multiplying the weight of ingredients used in cooking the food by the proportion of total prepared food consumed by the index child.
Weighed food record
In the two schools participating in the SFP, lunch was consumed by the children at school and was usually served by the kitchen staff before 12.00 hours. Therefore, weighed food records were collected from Monday to Friday to assess the food and nutrient intakes from the school lunch. For the 20 % of children who had a repeated 24 hR, a second weighed food record was collected on a non-consecutive day to match their day of repeated 24 hR. Weighed food records were collected on days preceding the scheduled 24 hR for each child. All raw ingredients used in preparing the school lunch for a particular day were weighed using a digital kitchen scale (HD-801 model; Yuyao Fuming Electrical Appliance Co., Ltd), precise to 1 g and a maximum capacity of 3 kg. Bulk food ingredients were weighed using a platform scale (Camry FD-250; Zhongshan Camry Electronic Co., Ltd), precise to 500 g and a maximum capacity of 250 kg. The weight of the total food cooked, the quantity served to each child and the quantity left over from each child's meal (when applicable) were determined to derive the proportion consumed by the child from the total dish prepared at school. Sharing of meals with peers was not a problem as all children in participating schools received the school lunch. All other meals not consumed in school were considered as home consumption for SFP participants, while all meals consumed were considered as home consumption for non-SFP participants.
Anthropometric measurements
The weight and height of children were measured according to standard procedures( Reference Cogill 20 , 21 ). Weight was measured precise to 0·1 kg with an electronic scale (Uniscale; Seca GmbH). A known weight (20 kg) was used to calibrate the scale on each measurement day. A microtoise (Bodymeter 208; Seca GmbH) was used to measure the height of children precise to 0·1 cm. For both weight and height, an average of two measurements was taken. The ages of children were determined using the date of birth (from a verifiable document) and the date of measurement. In the absence of verifiable documents, parents/caregivers estimated the age based on another child's records or an event on the traditional calendar.
Blood sample collection
From each child, venous blood (6 ml) was drawn through venepuncture. One-third (2 ml) of the whole blood was transferred into EDTA-coated vacutainers (Becton-Dickinson Diagnostics) and used for the determination of Hb concentration on the same day. The remaining 4 ml of blood was stored in a plain tube without anticoagulant at ambient temperature. Serum was separated at room temperature at 500 g for 10 min (Hettich GmbH) and stored at − 80°C (Thermo Fisher Scientific). Serum samples were transported on dry ice to Germany via The Netherlands for the analysis of serum ferritin (SF), soluble transferrin receptor (sTfR) and C-reactive protein (CRP).
Data analysis
Household hunger score
Following the standard coding, each of the three items in the HHS was coded 0, 1 or 2 corresponding to hunger frequencies of ‘never’, ‘rarely or sometimes’ or ‘often’. This yielded total scores ranging from 0 to 6 based on which households were categorised into three standard groups: 1 = little/no household hunger (HHS ≤ 1); 2 = moderate household hunger (HHS 2–3); 3 = severe household hunger (HHS = 4–6)( Reference Ballard, Coates and Swindale 17 ).
Food composition and nutrient intake calculation
The calculation of nutrient intake was based on a food composition database primarily created using nutrient values from the West African Food Composition Table (WAFCT)( Reference Stadlmayr, Charrondiere and Enujiugha 22 ). In case of missing foods (twenty-one of 138 foods), the following food composition tables were used in the order indicated: Mali Food Composition Table( Reference Barikmo, Ouattara and Oshaug 23 ); the United States Department of Agriculture National Nutrient Database for Standard Reference( 24 ); the Ghana Food Composition Table( Reference Eyeson and Ankrah 25 ). When food values were taken from the Ghana Food Composition Table, values of missing nutrients (vitamins and some minerals) were updated with those of close substitutes from the WAFCT. Phytate values were taken from the International Minilist( Reference Calloway and Murphy 26 ). For Corn Soy Blend Plus (CSB+) consumed under SFP, nutrient content values were obtained from the World Food Programme( 27 ). Where appropriate, yield( Reference Stadlmayr, Charrondiere and Enujiugha 22 ) and nutrient retention factors( 24 , Reference Vásquez-Caicedo, Bell and Hartmann 28 ) were applied to account for nutrient losses during cooking before computing nutrient intake values. The Atwater general factors for carbohydrate, protein and fat and the recommended metabolisable energy value for dietary fibre in an ordinary diet (8·4 kJ/g) were used for calculating energy intake( 29 ). Total vitamin A (retinol activity equivalent) was calculated as the sum of retinol and 1/12 β-carotene( Reference Stadlmayr, Charrondiere and Enujiugha 22 ). The food consumption data were analysed using the VBS Food Calculation System, version 4 (BaS Nutrition Software). Using the National Research Council method, data on dietary intake from the 24 hR were adjusted for day-to-day variations to obtain the estimated usual intake values for the children( 30 ). Individual foods were categorised into thirteen food groups( Reference Stadlmayr, Charrondiere and Enujiugha 22 ). Due to implausible dietary intake (energy intake >20 000 kJ), thirty-one (8 %) children were not included in the dietary intake analysis.
Energy and nutrient intake adequacy calculation
Estimated energy requirement was calculated separately for each child by multiplying the estimated energy requirement per kg body weight per d by the child's weight assuming a moderate physical activity level( 31 ). Similarly, sex- and age-specific safe levels of protein intake/kg body weight per·d were multiplied by the weight of the child to determine the safe levels of protein intake for each child( 32 ). To assess the prevalence of adequate or inadequate intake, each child's adjusted energy and protein intake values were compared with their respective calculated requirements.
The probabilities of adequacy (PA) for vitamins A, C, B12 and folate, Zn and Ca were calculated using their respective estimated average requirement and distribution values( 33 – 35 ). Because the distribution of Fe requirement is skewed, we used the PA values derived by the Institute of Medicine( 33 ), but adjusted them for 5 % bioavailability to reflect the inhibitory nature of the predominantly cereal-based diet in rural northern Ghana. Similarly, the estimated average requirement for Zn was adjusted for low (15 %) bioavailability( 36 ). The mean probability of adequacy, a summary measure of micronutrient adequacy, was computed from PA of all the seven micronutrients investigated in the study.
Anthropometry
Anthropometric Z-scores were calculated using AnthroPlus (version 1.0.3; WHO). Underweight, stunting and thinness were defined as weight-for-age, height-for-age and BMI-for-age Z-scores < − 2 sd, respectively( Reference Cogill 20 , 21 ).
Biochemical analysis
The cyanmethaemoglobin method (using a colorimeter) was used to measure the Hb concentration of schoolchildren( Reference Zwart, van Assendelft and Bull 37 ). Measurements of serum parameters (ferritin, sTfR and CRP) were done in an accredited laboratory (Labor Centrum Nordhorn, Nordhorn, Germany). The concentration of ferritin was measured using the ElectroChemiLuminescence Immunoassay on a Roche E170 clinical analyser (Roche Diagnostics) with intra-assay and inter-assay variations of 2–5 %. The concentration of sTfR was measured using the Ramco ELISA kit (Ramco Laboratories, Inc.) with intra-assay and inter-assay variations ranging from 5 to 8 %. Turbidimetry was used to measure CRP on a Beckman Coulter Synchron clinical analyser (Beckman Coulter) with combined intra-assay and inter-assay variations ranging from 1·6 to 3·5 %.
Anaemia was defined as Hb concentrations < 115 g/l for children aged < 12 years and as concentrations < 120 g/l for children aged ≥ 12 years. Fe deficiency (ID) was defined as SF concentrations < 15 μg/l and/or sTfR concentrations >8·5 mg/l (Ramco Laboratories, Inc.) and Fe-deficiency anaemia (IDA) as concurrent anaemia with ID. Inflammation was defined as CRP concentrations >10 mg/l. Body Fe concentration was calculated using Cook's formula( Reference Cook, Flowers and Skikne 38 ).
Statistical analysis
Data entry was done using Epi Info for Windows version 3.2.1 (CDC). Data cleaning and analysis were done in SPSS version 18.0 (SPSS, Inc.) and SAS version 9.2 (SAS Institute, Inc.). The distribution of data was checked by visual examination of Q–Q plots and normal-curve-fitted histograms and also tested for normality using the Kolmogorov–Smirnov test. Nutrient and Fe status variables that were not normally distributed were log-transformed and the transformed variables were used in subsequent analysis. ANOVA was used to generate a within-person day-to-day variance component, which was used to adjust energy and nutrient intakes.
Descriptive statistics were computed for background and household characteristics of children, and Pearson's χ2 test and independent-samples t test were used to test between-group differences in proportions and means, respectively. ANCOVA was used to test differences in the mean adjusted nutrient intake and Hb concentration values as well as serum Fe parameters between the two groups while controlling for age, household size and nutritional status (BMI-for-age Z score; BAZ). Differences in the prevalence of anaemia, inflammation, ID, IDA and inadequate nutrient intakes between the two groups were checked using Cox regression( Reference Barros and Hirakata 39 , Reference Coutinho, Scazufca and Menezes 40 ). Where appropriate, child and household characteristics were included in the regression model as covariates. In all analyses, P< 0·05 was the default value for an outcome to be considered statistically significant.
Results
Background characteristics of schoolchildren
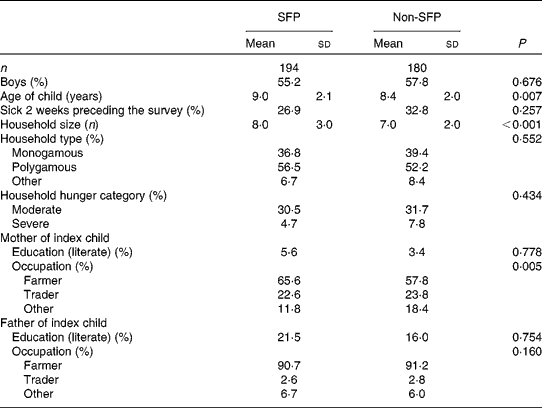
Characteristic of the study area, more than 55 % of the children in both school feeding and non-school feeding groups were boys. The average age of children in both groups was 8·5 (sd 2) years; however, SFP participants were, on average, 6 months older than non-SFP participants (P =0·007). There was no significant difference in the proportion of children who were reported ill during the 2 weeks preceding the survey between the two groups (P= 0·257). Household size was larger for SFP participants than for non-SFP participants (P< 0·001). More than half of the children in both groups were from polygamous households. There was no difference in the proportion of households that reported moderate or severe hunger between the two groups (P= 0·434). In both groups, the majority of parents/caregivers were illiterate and engaged in farming as their main occupation (Table 1).
Table 1 Background characteristics of school feeding programme (SFP) and non-school feeding programme (non-SFP) participants in northern Ghana (Mean values, standard deviations and percentages)
Food consumption patterns at home and school
At home, the three main meals served to children in both groups consisted of maize porridge (koko) with or without sugar served as breakfast and tuo zaafi – a thick/stiff maize porridge – served as lunch and dinner with varying vegetable soups. At the time of the survey (post-harvest season), the most dominant soup consumed by more than 50 % of the children consisted of dried powdered okra with or without groundnut paste/groundnut flour. When available, green leaves such as amaranth, Hibiscus sabdariffa and baobab (fresh and dried) were also used to prepare the soups accompanying tuo zaafi. A key ingredient of the soups, among all households, was powdered amani (small dried whole fish also known as anchovies, eaten with bones).
For SFP participants, school lunch was more varied and based on a menu. The menu was generally planned around three main food items: rice; cowpea; multiple-micronutrient-fortified corn soya blend (CSB+ from the World Food Programme). Eggs, meat and fish were served at least once a week, while oranges were served twice a week. The following dishes were prepared with these food items: jollof rice (rice cooked in tomato sauce); waakye (rice and cowpeas cooked together and served with tomato sauce); gari and beans (roasted cassava grits and boiled cowpeas usually served with palm oil); tuo zaafi or gable (both prepared from CSB+).
Energy and nutrient intakes and their adequacies among schoolchildren
The median intakes of energy, macronutrients and selected minerals and vitamins were higher among SFP participants than among non-SFP participants (P< 0·001) and remained higher after controlling for child and household covariates. Whereas the contribution of fat to total energy intake was significantly higher among SFP participants (20 v. 16 %; P< 0·001), the contribution of carbohydrate to total energy intake was significantly higher among non-SFP participants (64 v. 65 %; P< 0·01). Even though the contribution of protein to total energy intake was similar (12 %) between the two groups, the proportion of total protein intake from animal sources, a measure of protein quality, was greater among SFP participants than among non-SFP participants (5 v. 3 %; P< 0·001). However, there was no difference (P= 0·268) in the proportion of total Fe intake from animal sources (meat, fish and poultry) between the two groups (Table 2).
Table 2 Energy, nutrient and phytate intakes of school feeding programme (SFP) and non-school feeding programme (non-SFP) participants in northern Ghana (Median values and interquartile ranges (IQR))

RAE, retinol activity equivalent.
* Meat, fish, eggs and milk.
† Meat and fish.
The proportion of SFP participants with energy intake below the requirement was significantly lower than that of non-SFP participants (4·7 v. 21·8 %; P< 0·001). However, none of the children in both groups had intake below the requirement for protein. The PA for Fe, Zn, Ca, and vitamins A and C, and folate were significantly higher (P< 0·001) among SFP participants than among non-SFP participants, with a mean PA of 0·61 (sd 0·13) among SFP participants compared with 0·18 (sd 0·11) among non-SFP participants (Table 3).
Table 3 Proportion of school feeding programme (SFP) and non-school feeding programme (non-SFP) participants with values below the estimated average requirement for energy and protein and probabilities of adequacy for selected micronutrients (Mean values and standard deviations)
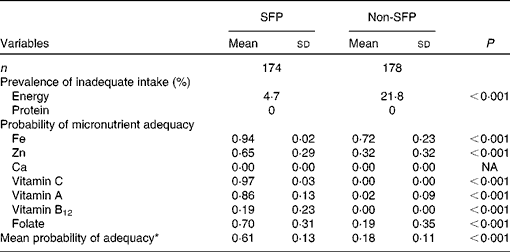
NA, not applicable.
* Computed from the PA values of micronutrients.
Home consumption and the contribution of school lunch to energy and nutrient intakes
There was no difference in energy, fat, carbohydrate, Ca, vitamin C and phytate intakes from home consumption between the two groups of children (P>0·05). Home intake was significantly higher among non-SFP participants for protein (P =0·041), Fe (P =0·011), Zn (P= 0·005) and vitamin A (P= 0·005). For energy, macronutrients and selected minerals, 22–37 % of the daily intake was contributed by the school lunch served to SFP participants. For vitamins A and C, however, >90 % of the daily intake was contributed by the school lunch (Table 4). The school lunch provided approximately 418 kJ more energy than home lunch (P< 0·001) and about 2 g more protein (18 (sd 1) v. 16 (sd 3) g; P< 0·001). The contribution of school lunch to the estimated average requirement for energy among SFP participants was significantly greater than that of home lunch among non-SFP participants, i.e. 37 (sd 7) v. 31 (sd 8) %; P <0·001. However, the contribution of school lunch to daily protein requirement among SFP participants did not differ from that among non-SFP participants, i.e. 88 (sd 17) v. 84 (sd 26) %; P= 0·096.
Table 4 Difference in home consumption between school feeding programme (SFP) and non-school feeding programme (non-SFP) participants and the contribution of school lunch to nutrient intakes among SFP participants (Median values and interquartile ranges (IQR))

RAE, retinol activity equivalent.
* Including foods bought outside home and consumed by children.
Relative contribution of individual foods and food groups to energy and nutrient intakes
In Fig. 1, the five topmost individual foods contributing to ≥ 70 % of the intake of energy, selected nutrients and anti-nutrients related to Fe absorption are shown. Except for vitamin C, maize was the main source of intake of total energy and selected nutrients. The relative contribution of maize to the intake of energy and selected nutrients ranged from 43 to 70 % for non-SFP participants and from 30 to 60 % for SFP participants. Cowpeas and corn soya blend (CSB+ from the World Food Programme) were additional sources of energy and nutrient intakes for SFP participants.

Fig. 1 The top five foods contributing to (a) energy, (b) protein, (c) iron, (d) zinc, (e) vitamin C and (f) phytate intakes among school feeding programme (SFP, ■) and non-school feeding programme (non-SFP, ![]() ) participants in northern Ghana. CSB+, Corn Soya Blend Plus; dawadawa, local condiment made from fermented African locust bean (Parkia biglobosa seeds); HS, Hibiscus sabdariffa.
) participants in northern Ghana. CSB+, Corn Soya Blend Plus; dawadawa, local condiment made from fermented African locust bean (Parkia biglobosa seeds); HS, Hibiscus sabdariffa.
For both groups of children, the main food groups that contributed to dietary intake were cereals (maize, rice and sorghum), vegetables (dried okra and green leaves), nuts (groundnuts) and fish (amani). Food groups such as meat, eggs and fruits were rarely consumed by non-SFP participants (Fig. 2). SFP participants received meat at school twice a week, but the average quantity per serving was < 10 g/d. The overall dietary diversity (number of different food groups consumed out of the thirteen food groups) among SFP participants was greater than that among non-SFP participants, i.e. 8·5 (sd 0·9) v. 6·2 (sd 1·1); P< 0·001.

Fig. 2 Proportion of school feeding programme (SFP, ■) and non-school feeding programme (non-SFP, ![]() ) participants in northern Ghana consuming foods from thirteen food groups.
) participants in northern Ghana consuming foods from thirteen food groups.
Eating moments and portion sizes of meals of schoolchildren
Almost all children in both groups ate during each of the three main eating moments per d: breakfast; lunch; dinner. Whereas a higher proportion of SFP children consumed a meal before the main breakfast meal (36 v. 25 %; P= 0·018), the reverse was true for children who ate a meal before lunch (13 v. 40 %; P< 0·001). Compared with only 20 % of the non-SFP children, almost every SFP child consumed a meal before the main dinner meal. For SFP participants, the meal before the main dinner meal could best be described as a second lunch (Fig. 3) after the school lunch. On the average, SFP participants had about one more eating moment (meal) compared with non-SFP participants (4·5 v. 3·8; P< 0·001).

Fig. 3 Proportion of school feeding programme (SFP, ■) and non-school feeding programme (non-SFP, ![]() ) participants in northern Ghana who ate meals across the six daily eating moments.
) participants in northern Ghana who ate meals across the six daily eating moments.
Except for ‘lunch’ and the meal ‘before dinner’, the average portion sizes of meals during all other eating moments in a day were similar between SFP participants and non-SFP participants (data not shown). The median portion size of lunch for non-SFP participants (which was taken at home) was significantly greater than that for SFP participants (which was taken at school), i.e. 1037 v. 456 g; P< 0·001. Conversely, the median portion size of the meal ‘before dinner’ was significantly greater for SFP participants than for non-participants, i.e. 962 v. 508 g; P< 0·001. The caregivers of SFP participants indicated that even though their children ate lunch at school, they still served them the lunch that was prepared at home. It should be noted that the portion size of the meal before dinner for SFP participants is similar to the portion size of the home lunch for non-SFP participants.
Iron and nutritional status of schoolchildren
The mean Hb concentration of children was 100 (sd 16) g/l. SFP participants had 6 g/l higher Hb concentration than non-SFP participants (P< 0·001) even after controlling for household and child characteristics. There was no difference in the concentration of SF between the two groups. The concentration of sTfR was significantly lower among SFP participants than among non-SFP participants (P= 0·04). There was no difference in the calculated body Fe store between the two groups (P =0·08). There was no difference in the mean concentration of CRP and the proportion of children with inflammation between the two groups. The prevalence of anaemia was marginally lower (P =0·06) in SFP participants, while there was no significant difference in the prevalence of ID and IDA between the two groups. SFP participants were about 3 cm taller; however, the difference was not significant after controlling for age differences. Weight-for-age and height-for-age Z-scores were similar between the two groups. BMI-for-age Z-score was significantly higher for non-SFP participants (P =0·008). There was no difference in the prevalence of underweight, stunting and thinness between the two groups (Table 5).
Table 5 Iron and nutritional status of school feeding programme (SFP) and non-school feeding programme (non-SFP) participants in northern Ghana (Mean values and standard deviations; geometric means and interquartile ranges (IQR))

SF, serum ferritin; CRP, C-reactive protein; sTfR, soluble transferrin receptor; ID, Fe-deficiency; IDA, Fe-deficiency anaemia.
* Adjusted for background difference between the groups.
† n 175 for the SFP group and 161 for the non-SFP group.
‡ To convert body Fe from mg/kg to mmol/kg multiply by 0·0171( Reference Troesch, van Stuijvenberg and Smuts 74 ).
§ Defined as anaemia and SF concentrations < 15 μg/l and/or sTfR concentrations >8·5 mg/l.
∥ AnthroPlus software (version 1.0.3; WHO) allows weight-for-age calculation only for children aged 5–10 years old (n 136 for the SFP group and 139 for the non-SFP group).
Discussion
In the present study, we compared the energy and nutrient intakes and Fe and nutritional status of children in school feeding and non-school feeding schools. Energy and nutrient intakes and their adequacies were significantly higher among the school feeding participants than among the non-participants. However, there were no differences in the prevalence of Fe status indicators, underweight, stunting and thinness between the two groups.
The significantly higher intake of energy and nutrients among the school feeding participants is attributable to the supplementary effect of school meals( Reference Powell, Walker and Chang 41 – Reference Preston, Venegas and Rodríguez 45 ) and superior energy density of the school lunch( Reference Harding, Marquis and Colecraft 43 ). The school lunch was served before 12.00 hours, so children were probably hungry again by the time school closed at 14.00 hours and therefore were still able to eat a late lunch served at home. However, a different study has reported that school feeding rather replaces home consumption( Reference Martens 46 ). The school lunch also increased the diversity of meals of participating children, which has been shown to be related to increased quality and quantity of nutrient intakes in other studies( Reference Ferguson, Gibson and Opareobisaw 47 – Reference Torheim, Barikmo and Parr 51 ). In both groups, all children met their safe levels of intake for protein. However, the biological value of the protein may be low, given that only an average of 4 % is animal source protein and cereal protein is limiting in growth-supporting lysine. Even though we did not adjust for protein quality( Reference Blackburn and Southgate 52 , Reference Beaton, Calloway and Murphy 53 ), the digestibility of the protein may also be compromised given the high concentration of dietary fibre in the meals of both groups of children( Reference Blackburn and Southgate 52 ).
A few food items contributed to the better micronutrient intake among the school feeding participants: orange for vitamin C; fortified corn soya blend for Fe and vitamins A and C; palm oil for vitamin A. The multiple-micronutrient-fortified corn soya blend, in particular, appears to play a key role in increasing micronutrient intake and adequacy among the school feeding participants. This may thus indicate that adequate micronutrient intake may not be achieved by the mere provision of an extra meal through school lunch, but achieved by deliberate supply of micronutrient-dense foods( Reference Bundy, Burbano and Grosh 3 , Reference Alderman and Bundy 54 ). However, the bioavailability of the relatively higher amounts of Fe and Zn consumed among SFP participants may be reduced given the high phytate content of the diet in general( Reference Hurrell, Reddy and Juillerat 55 ) and the meagre contribution of animal protein to total dietary intake( Reference Hallberg, Bjorn-Rasmussen and Howard 56 ). Moreover, the oranges that were served (twice a week) with lunch, which could improve Fe bioavailability when consumed together with the school lunch( Reference Cook and Reddy 57 , Reference Teucher, Olivares and Cori 58 ), were rather taken home and often shared with younger siblings not in school. In the absence of school feeding, the probability of adequate micronutrient intake among schoolchildren is low (approximately 0·20). This to a large extent reflects the poor quality of diets at the household level as almost all meals consumed by the non-school feeding children were from home. In other studies, the micronutrient quality of cereal- and legume-based diets of rural African households has been reported to be poor and to contribute to the inadequate intake of bioavailable Fe( Reference Mitchikpe, Dossa and Ategbo 59 , Reference Neumann, Bwibo and Murphy 60 ).
The measurement of habitual dietary intake of individuals and groups remains a major challenge in dietary intake assessment( Reference RS and EL 18 ), but our use of 24 hR with a non-consecutive duplicate recall in a subsample has been recommended and shown to be adequate for such a measurement( Reference RS and EL 18 , Reference Murphy and Poos 61 , Reference Kigutha 62 ) and for the assessment of nutrient intake of schoolchildren( Reference Murphy, Gewa and Liang 63 ). Major sources of systematic bias in the use of 24 hR include under- or over-reporting of intake( Reference RS and EL 18 , Reference Margetts and Nelson 64 ), which could have resulted in the misclassification of nutrient intake adequacy. To minimise misreporting of food intake, mothers/caregivers were taken through a systematic multiple-pass procedure which aided recollection of foods and ingredients used in preparation of meals at home( Reference RS and EL 18 ). Out-of-home food intake may have been omitted by mothers/caregivers and may have led to an underestimation of nutrient intake( Reference Gewa, Murphy and Neumann 19 ). However, in this area, almost all meals are prepared and consumed at home and mothers/caregivers are fully involved in serving meals. Also, the presence of children during the interviews helped mother/caregivers to recall likely forgotten foods. We therefore believe that underestimation of nutrient intake was unlikely to have occurred.
The high prevalence of anaemia among these children is not unexpected. The study area is malaria endemic and malaria is among the leading causes of anaemia( Reference Ehrhardt, Burchard and Mantel 14 ) in this area. As the present study was conducted during the peak of malaria transmission (November–December), it is most likely that malaria contributed to the high prevalence of anaemia among these children( Reference Abizari, Moretti and Zimmermann 65 ). Notwithstanding the apparent contribution of malaria to anaemia, the high prevalence of IDA among these children may indicate that anaemia in a large proportion of these children is due to ID. The low prevalence of ID observed based on SF values alone rather than when combined with sTfR values highlights the difficulty of reliably measuring ID prevalence in settings where the prevalence of infections and infestations may be high. In an intervention trial in the same area, Abizari et al. found that baseline SF values (similar to the SF values observed in the present study) decreased in response to deworming and malaria treatment, thus giving credence to the use of sTfR values as the measure of Fe status in the present study. However, the low prevalence of elevated CRP (an acute-phase protein) among these children does not seem to indicate that SF values in the present study were possibly influenced by inflammation. Unlike CRP, α1-acid glycoprotein values increase and return to baseline values slowly( Reference Feelders, Vreugdenhil and Eggermont 66 ) and therefore it may be better to measure the concentrations of both CRP and α1-acid glycoprotein as composite markers of cross-sectional inflammation( Reference Abizari, Moretti and Zimmermann 65 , Reference Ayoya, Spiekermann-Brouwer and Stoltzfus 67 , 68 ), but the concentration of α1-acid glycoprotein was not measured in the present study.
It is tempting to suggest that the higher Hb concentration, better sTfR concentration and the relatively lower prevalence of anaemia among SFP participants may be associated with the overall better Fe content of the school lunch. However, the absence of a significant difference in SF and body Fe concentrations between the two groups coupled with a similar prevalence of ID and IDA does not support the observation that SFP may have contributed to Fe status. Fe status may also be influenced by non-dietary interventions. Health- and nutrition-related interventions associated with SFP, such as deworming, could have also contributed to the relatively better Hb and sTfR concentrations( Reference Adelman, Gilligan and Lehrer 4 ), but neither group of schools reported receiving deworming treatments in the 6 months preceding the study. In a randomised trial in the same area, it has been shown that school feeding coupled with deworming and malaria treatment significantly improves Hb concentrations and Fe status and reduces anaemia prevalence( Reference Abizari, Moretti and Zimmermann 65 ).
Contrary to our expectation, the higher energy and nutrient intakes among SFP participants did not result in a significant difference in nutritional status. The lack of effect of school feeding on nutritional status has also been observed elsewhere( Reference Danquah, Amoah and Steiner-Asiedu 69 , Reference Meme, Kogi-Makau and Muroki 70 ), and the main reason for the lack of effect has been ascribed to the substitution effect of SFP. In the present study, the reason for the lack of differences in nutritional status despite the absence of home lunch substitution remains unclear as further exploration was limited by the study design. Others have argued that SFP targeting children >2 years are unlikely to affect stunting in particular( Reference Bryce, Coitinho and Darnton-Hill 12 ). In settings where stunting prevalence is already high, there is increasing fear that the excess energy intake due to SFP could lead to obesity( Reference Black, Allen and Bhutta 1 ). The basis for such fear was not apparent in the present study. However, it is possible that the higher energy and nutrient intakes among SFP participants increased their activity levels at the expense of weight gains( Reference Grillenberger, Neumann and Murphy 71 ). Based on the differences between the estimated energy requirements for children and the adjusted energy intakes, it was found that a majority of the schoolchildren in both groups were in positive energy balance, but there was no evidence of positive energy balance in the nutritional status of the schoolchildren. On the other hand, it is also possible that the schoolchildren were more physically active and thus required more energy than what we estimated using a moderate physical activity level. The positive energy balance could have also been a result of caregivers overestimating the dietary intake of their children. However, if overestimation occurred, it is less likely to have affected the differences observed in nutrient intakes between the two groups as total energy intake from home consumption was not significantly different.
The evidence of the impact of SFP (e.g. government-run SFP) has been described as lacking rigour because of their non-experimental design( Reference Adelman, Gilligan and Lehrer 4 ). The design of the present study is also non-experimental, thus limiting the rigour of the inferences that can be drawn. The absence of measures of nutrient intakes and Fe and nutritional status for both groups at the start of the SFP and the non-random allocation of the pilot SFP did not allow us to isolate the impact of school lunch, even though we controlled for differences in child and household characteristics in the analysis. We matched SFP communities with non-SFP communities that were otherwise also qualified to receive school feeding, but were not enrolled at the time of the study. We examined our assumptions that intervention communities had starting status similar to their controls by comparing the outcomes of interest between all four SFP–non-SFP pairs (2 SFP × 2 non-SFP) to determine whether differences were consistently in favour of SFP. We observed consistent differences in favour of school feeding with respect to energy and nutrient intakes but not with respect to Fe and nutritional status, indicating that our assumption for similarity may not be strongly supported. However, it is possible that the paired comparisons lacked power to detect the consistent direction of effect because sample sizes were half of what the present study was powered for. Moreover, unobservable differences between communities may have altered the effects attributable to SFP( Reference Ahmed 72 ). It is recommended that studies that match schools to evaluate the effects of SFP include a large number of schools to account for differences in school and community characteristics( Reference Grantham-McGregor 73 ). It therefore remains a limitation of the present study that only two pairs of schools could be matched and included.
In conclusion, the present results indicate that school feeding is associated with higher intakes and adequacies of energy and nutrients, but not with the prevalence of Fe and nutritional status indicators. The results also indicate an important role for micronutrient-dense foods in the achievement of micronutrient adequacy within SFP.
Acknowledgements
The authors are grateful to the following teachers for their cooperation during the survey: Y. Abdul-Majeed (Wayamba primary school); A. Alaru (Wayamba primary school); A. A. Suhuyini (Jagbo primary school); I. Norgah (Tibung primary school); S. Inusah (Kpalgung primary school). They also thank L. van der Heijden (deceased) for her technical support during the training of research assistants and R. K. Adatsi of the Tamale Teaching Hospital for his technical support during blood sample collection and measurement of Hb concentration. A.-R. A., I. D. B. and M. A.-K designed the study; A.-R. A., C. B. and V. L. K. conducted and supervised fieldwork; M. A.-K., J. M.-H. and I. D. B. supervised field work; A.-R. A., C. B. and V. L. K. processed dietary data; A.-R. A. and I. D. B. analysed data; A.-R. A. wrote the first draft of the manuscript and all authors edited and approved the final version of the manuscript. The present study was supported by the Interdisciplinary Research and Education Fund of Wageningen University through the Tailoring Food Sciences to Endogenous Patterns of Local Food Supply for Future Nutrition Project.










