Introduction
Major depression is a highly prevalent psychiatric illness that occurs in approximately 7% of community-dwelling older adults (World Health Organization, 2017). In addition, another 10–15% of older adults are estimated to exhibit subclinical depressive symptoms, such as depressed mood, even in the absence of the clinical DSM-5 diagnosis of major depression (Kok & Reynolds, Reference Kok and Reynolds2017). Importantly, in older adults, longitudinal studies have found that the presence of depressive symptoms not only leads to functional impairment (Grabovich, Lu, Tang, Tu, & Lyness, Reference Grabovich, Lu, Tang, Tu and Lyness2010), but also substantially increases the risk for morbidity and mortality (Ariyo et al., Reference Ariyo, Haan, Tangen, Rutledge, Cushman, Dobs and Furberg2000; Schulz et al., Reference Schulz, Beach, Ives, Martire, Ariyo and Kop2000; Simonsick, Wallace, Blazer, & Berkman, Reference Simonsick, Wallace, Blazer and Berkman1995; Whooley & Browner, Reference Whooley and Browner1998). Given that roughly one in five adults in the United States is projected to be 60 years or older by 2030 (Centers for Disease Control and Prevention, 2013), there is an urgent need to identify the biological mechanisms that contribute to depressive symptoms in older adults, which could help improve prevention strategies to mitigate age-related risk for morbidity and mortality.
Poor sleep maintenance, as typically indexed by increased amounts of time spent awake in bed after initially falling asleep (also known as ‘wake time after sleep onset’ or WASO), is a common health complaint among older adults (Ohayon, Carskadon, Guilleminault, & Vitiello, Reference Ohayon, Carskadon, Guilleminault and Vitiello2004). Indeed, a large population-based survey by the National Sleep Foundation found that about two-thirds of community-dwelling older adults in the United States complain of poor sleep maintenance at least a few nights per week (National Sleep Foundation, 2003). Importantly, longitudinal data from community-dwelling, non-depressed older adults have identified poor sleep maintenance, as indexed by actigraphy-measured WASO, as a salient risk factor that increases the odds of developing depressive symptoms over a 5-year follow-up period (Maglione et al., Reference Maglione, Ancoli-Israel, Peters, Paudel, Yaffe, Ensrud and Stone2014). Moreover, evidence from a cross-sectional study in older adults revealed that the severity of depressive symptoms is specifically associated with an increased amount of WASO, but not with other dimensions of sleep, such as sleep efficiency, sleep onset latency, or total sleep time (Maglione et al., Reference Maglione, Ancoli-Israel, Peters, Paudel, Yaffe, Ensrud and Stone2012). Whereas these data suggest that an increased amount of WASO may be a key sleep component that drives the development of depressive symptoms in older adults, little is known about the mechanisms that underlie this relationship.
Inflammation (i.e. the excessive activation of the immune system) has recently emerged as a biologically plausible pathway by which poor sleep maintenance might translate to depressive symptoms in older adults. Indeed, both experimentally induced as well as naturally occurring disturbances of sleep maintenance have previously been shown to activate inflammatory pathways at transcriptional, cellular, and systemic levels (Irwin, Reference Irwin2015; Irwin & Opp, Reference Irwin and Opp2017). In turn, several cross-sectional (Doyle et al., Reference Doyle, de Groot, Harris, Schwartz, Strotmeyer, Johnson and Kanaya2013; Glaser, Robles, Sheridan, Malarkey, & Kiecolt-Glaser, Reference Glaser, Robles, Sheridan, Malarkey and Kiecolt-Glaser2003; Penninx et al., Reference Penninx, Kritchevsky, Yaffe, Newman, Simonsick, Rubin and Pahor2003; White, Kivimäki, Jokela, & Batty, Reference White, Kivimäki, Jokela and Batty2017) as well as prospective studies (Au, Smith, Gariépy, & Schmitz, Reference Au, Smith, Gariépy and Schmitz2015; Bell, Kivimäki, Bullmore, Steptoe, & Carvalho, Reference Bell, Kivimäki, Bullmore, Steptoe and Carvalho2017; Gimeno et al., Reference Gimeno, Kivimäki, Brunner, Elovainio, De Vogli, Steptoe and Ferrie2009; Matheny et al., Reference Matheny, Miller, Shardell, Hawkes, Lenze, Magaziner and Orwig2011; Niles, Smirnova, Lin, & O'Donovan, Reference Niles, Smirnova, Lin and O'Donovan2018; Valkanova, Ebmeier, & Allan, Reference Valkanova, Ebmeier and Allan2013; Zalli, Jovanova, Hoogendijk, Tiemeier, & Carvalho, Reference Zalli, Jovanova, Hoogendijk, Tiemeier and Carvalho2016) have suggested that raised levels of systemic inflammatory markers, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α or C-reactive protein (CRP), promote the development of depressive symptoms in older adults, although some studies have also yielded mixed or negative results regarding such associations (Adriaensen et al., Reference Adriaensen, Matheï, Vaes, van Pottelbergh, Wallemacq and Degryse2014a, Reference Adriaensen, Matheï, van Pottelbergh, Vaes, Legrand, Wallemacq and Degryse2014b; Bondy et al., Reference Bondy, Norton, Voss, Marks, Boudreaux, Treadway and Bogdan2021; Bremmer et al., Reference Bremmer, Beekman, Deeg, Penninx, Dik, Hack and Hoogendijk2008; Dimopoulos, Piperi, Psarra, Lea, & Kalofoutis, Reference Dimopoulos, Piperi, Psarra, Lea and Kalofoutis2008; Stewart, Rand, Muldoon, & Kamarck, Reference Stewart, Rand, Muldoon and Kamarck2009; van den Biggelaar et al., Reference van den Biggelaar, Gussekloo, de Craen, Frölich, Stek, van der Mast and Westendorp2007). Less is known about the role of endogenous interferon (IFN)-γ, a signature cytokine that is centrally involved in the cellular immune response to viral and microbial infections (Ivashkiv, Reference Ivashkiv2018; Miller, Maher, & Young, Reference Miller, Maher and Young2009; Pollard, Cauvi, Toomey, Morris, & Kono, Reference Pollard, Cauvi, Toomey, Morris and Kono2013; Zhang, Reference Zhang2007), in the link between poor sleep maintenance and depressive symptoms in older adults. Interestingly, some evidence suggests that poor sleep maintenance might alter various molecular components involved in IFN-γ-related pathways. For example, our group has previously demonstrated that experimentally induced disruption of sleep maintenance (i.e. partial sleep deprivation) leads to an increase in nuclear activation of signal transducer and activator of transcription 1 (STAT1), a cytoplasmatic protein that conveys the IFN-γ-related transcriptional signal (Irwin, Witarama, Caudill, Olmstead, & Breen, Reference Irwin, Witarama, Caudill, Olmstead and Breen2015). Moreover, observational studies found that self-reported difficulties maintaining sleep (Huang et al., Reference Huang, Huang, Chang, Kor, Chen and Wu2017) as well as clinical sleep disturbance (Jain et al., Reference Jain, Kahlon, Morehead, Lieblong, Stapleton, Hoeldtke and Levine2012) are both associated with elevated plasma levels of IFN-γ-inducible protein 10 (IP-10), which is a chemokine produced in response to IFN-γ stimulation. In turn, evidence from clinical studies implicates that alterations in IFN-γ-related pathways play a role in the development of depressive symptoms. Several clinical studies, for example, have demonstrated that patients with major depression present with increased levels of IFN-γ compared to non-depressed healthy controls (Dahl et al., Reference Dahl, Ormstad, Aass, Malt, Bendz, Sandvik and Andreassen2014; Maes et al., Reference Maes, Scharpé, Meltzer, Okayli, Bosmans, D'Hondt and Cosyns1994; Schmidt et al., Reference Schmidt, Lichtblau, Minkwitz, Chittka, Thormann, Kirkby and Himmerich2014; Seidel et al., Reference Seidel, Arolt, Hunstiger, Rink, Behnisch and Kirchner1995; Simon et al., Reference Simon, McNamara, Chow, Maser, Papakostas, Pollack and Wong2008), and various antidepressant medications are reported to suppress the production of IFN-γ (Brustolim, Ribeiro-dos-Santos, Kast, Altschuler, & Soares, Reference Brustolim, Ribeiro-dos-Santos, Kast, Altschuler and Soares2006; Kubera et al., Reference Kubera, Lin, Kenis, Bosmans, van Bockstaele and Maes2001; Maes et al., Reference Maes, Song, Lin, Bonaccorso, Kenis, De Jongh and Scharpé1999; Mohr, Goodkin, Islar, Hauser, & Genain, Reference Mohr, Goodkin, Islar, Hauser and Genain2001). Moreover, variations in the IFN-γ gene have previously been found to modify both depression risk (Oxenkrug et al., Reference Oxenkrug, Perianayagam, Mikolich, Requintina, Shick, Ruthazer and Summergrad2011) as well as the efficacy of antidepressant treatments (Myint et al., Reference Myint, Bondy, Baghai, Eser, Nothdurfter, Schüle and Schwarz2013). In addition, in clinical patient populations, the expression of IFN-γ ribonucleic acid (RNA) has been found to correlate with depressive symptoms (Kahl, Kruse, Faller, Weiss, & Rieckmann, Reference Kahl, Kruse, Faller, Weiss and Rieckmann2002). Paralleling these findings from human studies, work in animals has also demonstrated an involvement of IFN-γ-related pathways in depressive-like behavior (Campos, Vaz, Saito, & Teixeira, Reference Campos, Vaz, Saito and Teixeira2014; Kwant & Sakic, Reference Kwant and Sakic2004; Litteljohn, Nelson, & Hayley, Reference Litteljohn, Nelson and Hayley2014; O'Connor et al., Reference O'Connor, André, Wang, Lawson, Szegedi, Lestage and Dantzer2009). However, no prior study has examined whether poor sleep maintenance and alterations in IFN-γ biology act in concert to jointly contribute to depressive symptoms. Indeed, such joint effects might be particularly salient in older adults, a population that is well-known to show a high prevalence of sleep disturbance (Li, Vitiello, & Gooneratne, Reference Li, Vitiello and Gooneratne2018) as well as increases in inflammation (Campisi, Reference Campisi2013; Franceschi et al., Reference Franceschi, Bonafe, Valensin, Olivieri, De Luca, Ottaviani and De Benedictis2000; Franceschi & Campisi, Reference Franceschi and Campisi2014; Kennedy et al., Reference Kennedy, Berger, Brunet, Campisi, Cuervo, Epel and Sierra2014; Piber et al., Reference Piber, Olmstead, Cho, Witarama, Perez, Dietz and Irwin2019).
To address this gap, we objectively measured sleep maintenance as indexed by amounts of WASO during a night of naturalistic sleep with polysomnography (PSG) in a sample of community-dwelling, non-depressed older adults. The following morning, plasma levels of IFN-γ were evaluated, along with repeated assessments of self-reported depressed mood throughout the day as a cardinal depressive symptom. We hypothesized: (1) that poor sleep maintenance (as indexed by a greater amount of WASO) and (2) higher morning levels of IFN-γ would both be associated with greater severity of depressed mood throughout the following day; and (3) that the association between WASO and depressed mood would be moderated by IFN-γ. In addition, we explored whether IFN-γ would mediate the link between WASO and depressed mood (i.e. whether WASO would negatively influence mood through altering levels of IFN-γ).
Materials and methods
Procedures
Participants were community-dwelling, non-depressed older adults, who were recruited from the Sleep Health and Aging Research (SHARE) field study sample (Piber et al., Reference Piber, Olmstead, Cho, Witarama, Perez, Dietz and Irwin2019). Of 262 older adults who participated in the SHARE field study, 40 older adults agreed to participate in an additional sleep laboratory component (15.3% of the field study sample). Of this sample, complete sleep, immune, and behavioral data were available from 36 participants (13.7% of the field study sample). Participants were older adults aged 60 years and above, and free of current medical disorders, psychiatric disorders (including major depression as evaluated by the Structured Clinical Interview for DSM-IV Axis I Disorders), and sleep disorders (including insomnia disorder, sleep apnea, restless leg syndrome). Moreover, participants were free of medications with known effects on inflammation, such as anti-inflammatory, analgesic, or psychotropic medications.
For the current study, participants underwent a baseline assessment to evaluate various socio-demographic and clinical characteristics, followed by 2 weeks of outpatient actigraphy to confirm regular sleep patterns. Subsequently, participants were admitted to the sleep laboratory at the Clinical Translational Research Center (CTRC) at UCLA, where they underwent several nights of PSG, including an adaptation night (first night) and an evaluation night of naturalistic sleep (second night). In the morning after the evaluation night, blood samples were obtained for the assessment of plasma levels of IFN-γ, along with repeated evaluation of self-reported depressed mood throughout the day. Participants provided written consent prior to the enrollment. The study received oversight and approval from the UCLA Institutional Review Board (IRB#11-000656).
Baseline measures
During the baseline assessment, various socio-demographic and clinical characteristics were evaluated including the body mass index (BMI). The presence of medications other than the anti-inflammatory, analgesic, or psychotropic medications was evaluated using the chronic disease score (CDS) (Von Korff, Wagner, & Saunders, Reference Von Korff, Wagner and Saunders1992). Furthermore, given the known effects of physical activity on depressive symptoms (Mura & Carta, Reference Mura and Carta2013), amounts and patterns of physical activity were assessed using the Yale Physical Activity Survey for Older Adults (YPAS) Part II (Dipietro, Caspersen, Ostfeld, & Nadel, Reference Dipietro, Caspersen, Ostfeld and Nadel1993). The YPAS Part II evaluates the frequency and duration of physical activity in the last month across five distinct dimensions: vigorous activities, leisurely walking, moving, standing, and sitting. For each of these five dimensions, an index score is calculated by multiplying the responses by a specific weight factor. Subsequently, the five index scores are summed to determine a YPAS summary score.
Laboratory-based measures
Assessment of sleep measures
Following the baseline assessment, participants underwent 2 weeks of outpatient actigraphy to screen for regular sleep patterns with sleep periods between 10:00 p.m. and 7:00 a.m. Subsequently, participants were admitted to the sleep laboratory at CTRC, where they underwent all PSG procedures. Participants underwent several nights of PSG, including an adaptation night (first night) and an evaluation night of naturalistic sleep (second night). During the evaluation night, sleep maintenance was assessed, as indexed by WASO [i.e. the time (in minutes) spent awake in bed after the initial onset of sleep]. During the daytime, subjects were not allowed to consume caffeine or engage in any exhausting physical activity in order to reduce potential stress. Amounts of WASO were scored by a trained sleep technician according to the American Academy of Sleep Medicine (Berry et al., Reference Berry, Budhiraja, Gottlieb, Gozal, Iber, Kapur and Tangredi2012).
Assessment of inflammatory markers
Plasma levels of IFN-γ were evaluated at 8:00 a.m. following the evaluation night of PSG using the Meso Scale Discovery (MSD) MULTI-SPOT Assay System (Rockville, MD). Plasma samples were assayed in duplicate on a custom 5-plex from the Proinflammatory Panel 1 Human Kit. In addition to IFN-γ, MSD assays also evaluated plasma levels of TNF-α, IL-6, IL-8, and IL-10. Given the a priori hypothesis and specific focus on IFN-γ, analyses of TNF-α, IL-6, IL-8, and IL-10 were exploratory. Briefly, after a period of overnight fasting, blood samples were collected in EDTA tubes, and processed at 4 °C to plasma aliquots. Plasma aliquots were stored at −80 °C until assayed in a single batch. Assays were performed according to the manufacturer's protocol. ECL signals were measured on the MESO QuickPlex SQ 120 instrument (Rockville, MD), and the DISCOVERY WORKBENCH software (Rockville, MD) was used to generate a 4-parameter logistic fit curve. The mean intra-assay coefficient of variation (CV) for IFN-γ was less than 6%, and the mean inter-assay CV less than 12.2%, with similar CVs for other inflammatory markers.
Assessment of depressed mood
Throughout the day following the evaluation night of PSG, presence and severity of depressed mood was evaluated at 8:00 a.m. directly following the blood draw, and again at 2:00 p.m. and at 10:00 p.m. using the short-form of the Profile of Mood States Depression Subscale (POMS-D) (Baker, Denniston, Zabora, Polland, & Dudley, Reference Baker, Denniston, Zabora, Polland and Dudley2002). POMS-D is a self-administered questionnaire that assesses the extent to which a person experiences a depressed mood in the present moment, including feelings such as unhappy, sad, blue, hopeless, discouraged, miserable, helpless, and worthless. For each feeling, a subjective self-rating is collected on a 0–4 scale (0 = not at all; 1 = a little; 2 = moderately; 3 = quite a lot; 4 = extremely), resulting in a sum score with higher scores indicating greater severity of depressed mood. To account for circadian fluctuations of depressed mood, POMS-D scores were averaged across the 3 time points.
Statistical analyses
Data were analyzed using SPSS version 28 (IBM Inc., USA). To reduce skewedness of sleep and immune data, WASO and IFN-γ values were natural log-transformed. Prior to examining our main hypotheses, a series of partial correlations were computed to examine the relationships between WASO and assessed immune markers (i.e. IFN-γ, TNF-α, IL-6, IL-8, IL-10), while controlling for age, sex and BMI. To test the first hypothesis, that poor sleep maintenance as indexed by a greater amount of WASO would be associated with greater severity of depressed mood, a multivariate linear regression approach was used with WASO as an independent variable, and averaged POMS-D scores across the 3 time points as a dependent variable. To control for socio-demographic and clinical factors as well as levels of physical activity, we computed four different models: Model 1 was the unadjusted model (WASO only); Model 2 further included socio-demographic variables (Model 1 + age, sex, race, education); Model 3 further included clinical variables (Model 2 + BMI and CDS); finally, Model 4 further included levels of physical activity (Model 3 + YPAS summary score). To test the second hypothesis, that higher levels of IFN-γ would be associated with greater severity of depressed mood, an identical multivariate approach was used: Model 1 was the unadjusted model (IFN-γ only); Model 2 further included socio-demographic variables (Model 1 + age, sex, race, education); Model 3 further included clinical variables (Model 2 + BMI and CDS); Model 4 further included levels of physical activity (Model 3 + YPAS summary score). Using an identical regression modeling approach, exploratory analyses examined whether any of the other inflammatory markers, including TNF-α, IL-6, IL-8, and IL-10, were associated with the severity of depressed mood. To test the third hypothesis, that the association between WASO and depressed mood would be moderated by IFN-γ, we conducted a moderation analysis (online Supplementary Fig. S1). To additionally explore a potential mediation effect of IFN-γ on the relationship between WASO and depressed mood, we also conducted a mediation analysis (online Supplementary Fig. S2). Both moderation and mediation analyses were performed using the PROCESS macro for SPSS version 4.0 (Hayes, Reference Hayes2017). To test for moderation, we used WASO as an independent variable, IFN-γ as a moderator variable, and depressed mood (averaged POMS-D scores across the 3 time points) as an outcome variable. The PROCESS macro automatically created a WASO × IFN-γ interaction term and subsequently computed conditional follow-up simple slope tests, which allowed probing associations between WASO and depressed mood stratified by higher (i.e. one standard deviation above the mean) and lower IFN-γ (i.e. one standard deviation below the mean). To test for mediation, we used WASO as an independent variable, IFN-γ as mediator variable, and depressed mood (POMS-D scores across the 3 time points) as the outcome variable to conduct the following analyses: (1) depressed mood was regressed on WASO; (2) IFN-γ was regressed on WASO; (3) the depressed mood was regressed on IFN-γ; (4) depressed mood was regressed on both WASO and IFN-γ simultaneously. To estimate the indirect effect (i.e. whether WASO negatively influenced mood through altering levels of IFN-γ), the bootstrapping method was used with 10 000 bootstrapped resamples. The indirect effect was considered to be significant when the 95% confidence interval did not include 0.
Results
Sample characteristics
Table 1 summarizes the sample's baseline characteristics. The sample had a mean age of 72.1 ± 6.8 years (range: 61–86 years), with 52.8% females. Levels of physical activity in the last month were normal compared to other studies in older adult populations (Kruskall, Campbell, & Evans, Reference Kruskall, Campbell and Evans2004). There were no differences in socio-demographic variables, clinical characteristics or levels of physical activity between those who did (n = 40) and those who did not (n = 222) participate in the sleep laboratory component of the SHARE study (all p's > 0.05, online Supplementary Table S1). As shown in Table 2, the sample had modest disturbances of sleep maintenance, as indexed by a median WASO of 55 min, which was consistent with meta-analytical data on sleep characteristics in older adults (Ohayon et al., Reference Ohayon, Carskadon, Guilleminault and Vitiello2004).
Table 1. Baseline measures (n = 36)

YPAS, Yale Physical Activity Survey for Older Adults; s.d., standard deviation.
Table 2. Laboratory-based measures (n = 36)

WASO, wake time after sleep onset; IFN, interferon; POMS-D, Profile of Mood States – Depression Subscale; PSG, polysomnography; IQR, interquartile range.
a Assessed during the evaluation night of PSG.
b Assessed at 8:00 a.m. following the evaluation night of PSG.
c Assessed at 8:00 a.m., 2:00 p.m., and 10:00 p.m. following the evaluation night of PSG (POMS-D scores for each individual were averaged across the 3 time points).
Correlational analyses
Controlling for sex, age and BMI, partial correlational analyses showed that WASO correlated with IFN-γ (r = 0.35, p < 0.05), but not with any of the other immune markers, including TNF-α, IL-6, IL-8, and IL-10 (online Supplementary Table S2), which indicated that greater amount of WASO was associated with higher levels of IFN-γ (Fig. 1).

Fig. 1. Association between WASO and levels of IFN-γ. Shown is a scatterplot of WASO and IFN-γ levels. WASO was assessed during the evaluation night of PSG; IFN-γ was assessed at 8:00 a.m. following the night of PSG. WASO, wake time after sleep onset; IFN, interferon; PSG, polysomnography.
Associations between sleep maintenance and depressed mood
First, we examined whether poor sleep maintenance as indexed by WASO would be associated with greater severity of depressed mood throughout the following day, while controlling for socio-demographic, clinical and behavioral factors (online Supplementary Table S3). In the unadjusted model, a greater amount of WASO was associated with greater severity of depressed mood (Model 1: β = 0.43, p < 0.05). Importantly, this association remained significant after adjusting for age, sex, race, and education (Model 2: β = 0.42, p < 0.05), BMI and CDS (Model 3: β = 0.43, p < 0.05), as well as levels of physical activity (Model 4: β = 0.43, p < 0.05). Similar results were found for each of the three individual timepoints (data not shown).
Associations between IFN-γ and depressed mood
Second, we examined whether morning levels of IFN-γ would be associated with greater severity of depressed mood throughout the following day, while again controlling for socio-demographic, clinical and behavioral factors (online Supplementary Table S4). In the unadjusted model, heightened morning levels of IFN-γ were associated with greater severity of depressed mood (Model 1: β = 0.47, p < 0.01). Again, this association remained significant after adjusting for age, sex, race, and education (Model 2: β = 0.44, p < 0.01), BMI and CDS (Model 3: β = 0.46, p < 0.01), as well as physical activity (Model 4: β = 0.47, p < 0.01). Again, similar results were found for each of the three individual timepoints (data not shown). However, exploratory analyses found that none of the other inflammatory markers, including TNF-α, IL-6, IL-8, and IL-10, were associated with severity of depressed mood (data not shown).
Moderation analysis
Third, to examine whether WASO would be more strongly associated with severity of depressed mood among those with higher levels of IFN-γ, than among those with lower levels of IFN-γ, we conducted a moderator analysis. As hypothesized, results showed that the association between WASO and depressed mood was moderated by levels of IFN-γ, as indicated by a significant WASO × IFN-γ interaction [b = 2.59, 95% CI (1.03–4.15), p < 0.01]. Follow-up simple slope tests confirmed that the slope for one standard deviation above the mean levels of IFN-γ was positive and significantly different from zero [b = 3.67, 95% CI (1.80–5.54), p < 0.001], while the slope for one standard deviation below the mean level of IFN-γ was not significantly different from zero [b = 0.11, 95% CI (−1.42 to 1.64), p = 0.881]. Together, this indicated that a greater amount of WASO was more strongly associated with severity of depressed mood among older adults with higher levels of IFN-γ, than among those with lower levels of IFN-γ (Fig. 2).

Fig. 2. Moderation analysis. Shown are simple slopes with 95% confidence bands of the associations between WASO and depressed mood in subjects with higher (i.e. one s.d. above the mean; dashed line) and lower levels of IFN-γ (i.e. one s.d. below the mean; solid line). The x-axis shows mean centered values of natural log-transformed WASO values, the y-axis shows averaged POMS-D score across the 3 time points. WASO, wake time after sleep onset; IFN, interferon; POMS-D, Profile of Mood States – Depression Subscale; s.d., standard deviation.
Mediation analysis
To further evaluate the directional nature of relationships among WASO, IFN-γ, and depressed mood, we additionally conducted an exploratory mediation analysis (Fig. 3). Results showed significant associations between WASO and IFN-γ (a-path: β = 0.35, p < 0.05), between IFN-γ and depressed mood (b-path: β = 0.35, p < 0.05), between WASO and depressed mood while controlling for IFN-γ (direct effect/c′-path: β = 0.31, p < 0.05), and between WASO and depressed mood without controlling for IFN-γ (total effect/c-path: β = 0.43, p < 0.01). Interestingly, the association between WASO and severity of depressed mood was attenuated by about 28% when accounting for IFN-γ, which suggested a mediation effect, although the model was not statistically significant [i.e. the 95% confidence interval of the association between WASO and depressed mood via IFN-γ (indirect effect/ab-path) included zero].
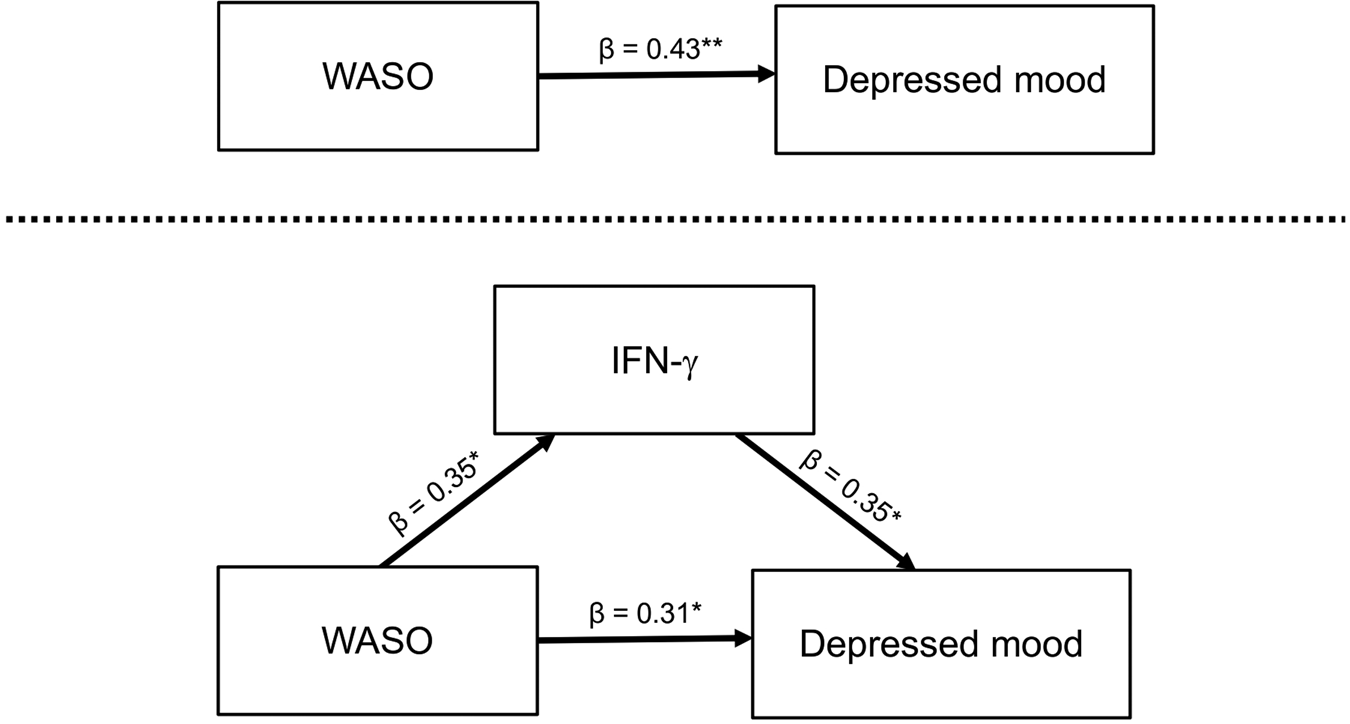
Fig. 3. Mediation analysis. Shown is the exploratory model of IFN-γ mediating the relationship between WASO and depressed mood. WASO was assessed during the evaluation night of PSG; IFN-γ was assessed at 8:00 a.m. following the night of PSG; the depressed mood was assessed at 8:00 a.m., 2:00 p.m., and 10:00 p.m. following night of PSG (POMS-D scores for each individual were averaged across the 3 time points). *p < 0.05, **p < 0.01. WASO, wake time after sleep onset; IFN, interferon; PSG, polysomnography.
Discussion
This is the first study to show that in community-dwelling, non-depressed older adults, objectively measured disturbance of sleep maintenance as evaluated by PSG as well as heightened morning plasma levels of IFN-γ are associated with greater severity of depressed mood throughout the following day. Moreover, our data show that the link between poor sleep maintenance and depressed mood is moderated by IFN-γ, such that poor sleep maintenance is more strongly associated with severity of depressed mood among older adults with higher IFN-γ, than among those with lower IFN-γ. Whereas correlational analyses showed that WASO was related to IFN-γ, we did not find an ‘indirect’ (i.e. mediation) effect of IFN-γ on the relationship between WASO and depressed mood.
First, our data showed that even modest disturbance of sleep maintenance as assessed by PSG was associated with greater severity of self-reported depressed mood throughout the following day. These observations were consistent with previous data from community-dwelling older adults which demonstrated that increases in WASO assessed by actigraphy are associated with severity of depressive symptoms (Maglione et al., Reference Maglione, Ancoli-Israel, Peters, Paudel, Yaffe, Ensrud and Stone2012, Reference Maglione, Ancoli-Israel, Peters, Paudel, Yaffe, Ensrud and Stone2014). Moreover, our findings are in line with experimental data which have demonstrated that experimental disruption of sleep maintenance (i.e. sleep deprivation) induces depressed mood as assessed by POMS-D (Scott, McNaughton, & Polman, Reference Scott, McNaughton and Polman2006). In addition, our findings parallel longitudinal data which have shown that the presence of clinical sleep disturbance is associated with an increased risk to develop major depression in both the general population (Baglioni et al., Reference Baglioni, Battagliese, Feige, Spiegelhalder, Nissen, Voderholzer and Riemann2011) and in older adults (Cho et al., Reference Cho, Lavretsky, Olmstead, Levin, Oxman and Irwin2008; Lee et al., Reference Lee, Cho, Olmstead, Levin, Oxman and Irwin2013).
Second, our data showed that heightened morning levels of IFN-γ were associated with greater severity of depressed mood throughout the following day. While no prior study specifically examined putative associations between levels of IFN-γ and depressed mood in older adults, our results are partially paralleled by previous clinical studies on the role of IFN-γ in major depression. Indeed, several studies have demonstrated that patients with major depression show increased production of IFN-γ as compared to non-depressed healthy controls (Dahl et al., Reference Dahl, Ormstad, Aass, Malt, Bendz, Sandvik and Andreassen2014; Maes et al., Reference Maes, Scharpé, Meltzer, Okayli, Bosmans, D'Hondt and Cosyns1994; Schmidt et al., Reference Schmidt, Lichtblau, Minkwitz, Chittka, Thormann, Kirkby and Himmerich2014; Seidel et al., Reference Seidel, Arolt, Hunstiger, Rink, Behnisch and Kirchner1995; Simon et al., Reference Simon, McNamara, Chow, Maser, Papakostas, Pollack and Wong2008), although meta-analytical work concluded that patients with major depression show comparable (Dowlati et al., Reference Dowlati, Herrmann, Swardfager, Liu, Sham, Reim and Lanctot2010; Liu, Ho, & Mak, Reference Liu, Ho and Mak2012) or even lower levels of IFN-γ compared to non-depressed individuals (Köhler et al., Reference Köhler, Freitas, Maes, de Andrade, Liu, Fernandes and Carvalho2017). In addition, polymorphism in the IFN-γ gene in human subjects has been linked to increased activity of the indoleamine-2,3-dioxygenase (IDO) (Raitala, Pertovaara, Karjalainen, Oja, & Hurme, Reference Raitala, Pertovaara, Karjalainen, Oja and Hurme2005), a key enzyme that catalyzes tryptophan to kynurenine, which in turn is known to contribute to the development of inflammation-driven depressive symptoms (Dantzer, O'Connor, Lawson, & Kelley, Reference Dantzer, O'Connor, Lawson and Kelley2011; Vancassel, Capuron, & Castanon, Reference Vancassel, Capuron and Castanon2018). Paralleling these findings from human studies, also data from mechanistic animal models have proposed a pathway in which IFN-γ induces depressive-like behavior through increasing IDO activation (O'Connor et al., Reference O'Connor, André, Wang, Lawson, Szegedi, Lestage and Dantzer2009). Thus, our data extend previous work on the role of IFN-γ for mood regulation by demonstrating that higher morning levels of IFN-γ are associated with greater severity of self-reported depressed mood throughout the day in a non-clinical population of non-depressed older adults. Unlike IFN-γ, none of the other assessed inflammatory markers, including TNF-α, IL-6, IL-8, or IL-10, were associated with severity of depressed mood throughout the day. This was surprising, given that previous work has linked increased levels of inflammatory markers to depressive symptoms in older adults (Au et al., Reference Au, Smith, Gariépy and Schmitz2015; Bell et al., Reference Bell, Kivimäki, Bullmore, Steptoe and Carvalho2017; Doyle et al., Reference Doyle, de Groot, Harris, Schwartz, Strotmeyer, Johnson and Kanaya2013; Gimeno et al., Reference Gimeno, Kivimäki, Brunner, Elovainio, De Vogli, Steptoe and Ferrie2009; Glaser et al., Reference Glaser, Robles, Sheridan, Malarkey and Kiecolt-Glaser2003; Matheny et al., Reference Matheny, Miller, Shardell, Hawkes, Lenze, Magaziner and Orwig2011; Penninx et al., Reference Penninx, Kritchevsky, Yaffe, Newman, Simonsick, Rubin and Pahor2003; Valkanova et al., Reference Valkanova, Ebmeier and Allan2013; Zalli et al., Reference Zalli, Jovanova, Hoogendijk, Tiemeier and Carvalho2016), although some studies have also yielded mixed or negative results regarding such associations (Adriaensen et al., Reference Adriaensen, Matheï, Vaes, van Pottelbergh, Wallemacq and Degryse2014a, Reference Adriaensen, Matheï, van Pottelbergh, Vaes, Legrand, Wallemacq and Degryse2014b; Bondy et al., Reference Bondy, Norton, Voss, Marks, Boudreaux, Treadway and Bogdan2021; Bremmer et al., Reference Bremmer, Beekman, Deeg, Penninx, Dik, Hack and Hoogendijk2008; Dimopoulos et al., Reference Dimopoulos, Piperi, Psarra, Lea and Kalofoutis2008; Stewart et al., Reference Stewart, Rand, Muldoon and Kamarck2009; van den Biggelaar et al., Reference van den Biggelaar, Gussekloo, de Craen, Frölich, Stek, van der Mast and Westendorp2007).
Finally, confirming our third hypothesis, our data showed that the association between WASO and depressed mood was moderated by IFN-γ, such that a greater amount of WASO was more strongly related to the severity of depressed mood among older adults with higher levels of IFN-γ, than among those with lower levels of IFN-γ. Although our exploratory mediation analysis did not demonstrate a significant indirect (i.e. mediation) effect, the association between WASO and severity of depressed mood was substantially attenuated when accounting for IFN-γ, which nonetheless suggested a ‘mediation-like’ effect. This lack of a significant mediation effect might have been due to the small sample size and the related limitations in statistical power. Thus, future research should examine IFN-γ mediation pathways on the link between sleep disturbance and mood regulation in a larger sample. Although no prior study examined moderation and mediation effects of IFN-γ on associations between poor sleep maintenance and depressed mood, our findings are paralleled by previous research that linked sleep disturbance to increased activity of STAT1 (Irwin et al., Reference Irwin, Witarama, Caudill, Olmstead and Breen2015) and IP-10 (Huang et al., Reference Huang, Huang, Chang, Kor, Chen and Wu2017; Jain et al., Reference Jain, Kahlon, Morehead, Lieblong, Stapleton, Hoeldtke and Levine2012). Moreover, our data support our previously conceptualized two-hit model of depression risk (Irwin & Piber, Reference Irwin and Piber2018), in which we hypothesized that sleep disturbance and increased inflammation serve as mutual vulnerability factors for depression risk, mirroring longstanding clinical observations that the risk of depression is increased when sleep disturbance and states of increased inflammatory activity coincide. However, we found no correlational association between WASO and any of the other inflammatory markers, including IL-6, IL-8, IL-10, and TNF-α.
While the present study is the first analysis to evaluate the joint contributions of poor sleep maintenance and IFN-γ biology to mood regulation in older adults, our work does not come without limitations. Our sample size was rather small which might have limited statistical power, although we observed a consistent statistical significance in the analyses including moderation analysis. Another limitation of our study is the lack of racial and ethnic diversity, given that the majority of subjects self-identified as white and non-Hispanic/non-Latino. Thus, future research on the role of sleep disturbance, inflammation and mood regulation should include a broader range of diversity.
Taken together, our data suggest that sleep disturbance and increased levels of IFN-γ might jointly contribute to depressed mood in older adults. Indeed, our findings provide support for a moderating role of IFN-γ on the relationship between WASO and depressed mood, which implicates that higher levels of IFN-γ might serve as a biological vulnerability factor that augments the effects of sleep disturbance on mood regulation in older adults. Even though our exploratory mediation model was not statistically significant, the inclusion of IFN-γ in the model substantially reduced the association between sleep disturbance and depressive symptoms; the absence of significant mediation may be due to the small sample size and the respective lack of statical power. Indeed, such hypothetical mediation effect of IFN-γ on the relationship between WASO and depressed mood could follow various pathways. For example, longer periods of wakefulness during the night might subsequently raise levels of IFN-γ, which in turn might alter thresholds for affect responses. Alternatively, individuals with higher baseline levels of IFN-γ might be particularly vulnerable to the potentially depressing effects of sleep disturbance. Conversely, lower levels of IFN-γ might possibly serve as a resilience factor that could ameliorate the adverse effects of sleep disturbance on mood in older adults. However, to evaluate and test these hypotheses, further research in larger samples (and also clinical populations) with more repeated measures is needed. Indeed, gaining insight into the specific aspects of how sleep disturbance and IFN-γ biology jointly contribute to depressed mood in older adults could help improve prevention strategies to mitigate age-related risk for morbidity and mortality.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722000113
Financial support
This work was supported by the Max Kade Foundation, the National Institutes of Health (Grant No. R01 AG034588 to MRI), the UCLA Cousins Center for Psychoneuroimmunology at the Semel Institute for Neuroscience. In addition, Dr Piber is a participant of the Berlin Institute of Health (BIH) Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the BIH.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








