Introduction
Foodborne diseases (FBD) are illnesses caused by ingesting food contaminated with various aetiologies, including microorganisms, chemical substances, mushroom toxins, poisonous animals and plants [Reference Russini1]. FBD can cause a range of health issues, including food poisoning, intestinal infections, and metabolic disorders. These diseases impose a considerable disease burden on every country and pose substantial challenges to public health [Reference Kirk2].
In 2015, the World Health Organisation (WHO) published the first comprehensive assessment report on FBD. This report revealed that approximately one in 10 people get sick each year due to the consumption of contaminated food, leading to approximately 420,000 fatalities [3]. In China, an estimated 7,073 foodborne outbreaks occurred in 2020, resulting in approximately 37,454 reported cases and 143 deaths [Reference Li4].
In a foodborne outbreak, it is critical to rapidly identify the aetiology and specific food responsible for the outbreak. The longer an outbreak lasts, the greater its impact on public health and the economy [Reference Lai5]. However, identifying the sources of these epidemiological characteristics remains challenging [Reference Magalhães6]. Research has indicated that only approximately 50% of foodborne outbreaks have identified aetiologies, and data on outbreak characteristics that assist in identifying the contributing factors are limited [Reference Brown7]. Investigations and subsequent analyses of outbreaks have provided valuable insights into the epidemiological characteristics. This information can aid in the reduction and prevention of future foodborne outbreaks. Additionally, various factors, including geography, climate, local diet, lifestyle, and sanitary conditions, significantly affect the relative frequency of foodborne outbreaks [Reference Li8].
The Foodborne Diseases Surveillance and Reporting System (FDSRS) and Foodborne Outbreaks Surveillance System (FDOSS) have been established in China to collect, analyse, and report data related to FBD and foodborne outbreaks [Reference Li9]. These systems can collect data from various sources, including hospitals, the Centers for Disease Control and Prevention (CDC), laboratories, regulatory agencies, and food enterprises. In the Zhejiang Province, FDSRS and FDOSS have been fully implemented since 2010 and comprise active and passive surveillance [Reference Liu10]. This study analysed data related to foodborne outbreaks in Wenzhou, a seashore city in Zhejiang, China, to comprehensively summarise the characteristics of foodborne outbreaks from 2012 to 2022. Wenzhou has a subtropical climate characterised by warm and humid weather. Temperatures in the third quarter peak, often exceeding 30.0 °C and occasionally reaching 38.0 °C to 40.0 °C. These elevated temperatures and increased rainfall result in higher humidity, particularly during July and August, when humidity can exceed 80.0% or even increase further. The unique geographical location and climate of Wenzhou are closely linked to outbreaks. Basic epidemiological situations were characterised, including the general characteristics of the outbreaks, population distribution, time and regional distribution, setting, aetiology, and food. Further analysis may improve the identification of foods responsible for and causes of outbreaks. Ultimately, these statistical analyses contribute to a better understanding of the transmission rules and characteristics of FBD and provide technical support for ongoing control and prevention.
Methods
Definitions
Foodborne outbreaks are defined as two or more similar diseases resulting from consuming a common food source [Reference Dewey-Mattia11]. In 1996, the Ministry of Health published the diagnostic criteria and principles for managing foodborne outbreaks of different aetiologies, which continue to be in use [12]. The year is divided into four quarters: the first quarter encompasses the months of January to March, the second quarter includes April to June, the third quarter spans July to September, and the fourth quarter covers October to December. Outbreaks were assigned to quarters based on the date of exposure. The terms used in this study are defined in the Supplementary Material.
Data source
The official statistics used in this study were obtained from the FDOSS of Wenzhou. Medical institutions were required to report FBD case information to the FDSRS within two working days after the FBD case was treated. The CDC reported case information to this system daily, and once two or more suspected cases were found, the information was verified promptly. In addition, according to the Food Safety Law, the units where suspected foodborne outbreaks occurred were responsible for reporting them to the CDC. After an outbreak was confirmed, the CDC organised professional staff to conduct epidemiological investigations, which included the number of cases, hospitalisations and deaths, onset date, outbreak setting, aetiology, clinical features, and foods served. An epidemiological investigation report was uploaded to FDOSS in a uniform format. Population data from 2012 to 2022 were obtained from the Statistical Yearbook of Wenzhou.
Statistical analysis
All statistical analyses were conducted using the Statistical Analysis System (SAS), version 9.4. A chi-square test was used to compare the differences in attack rates among annual quarters and aetiologies. A two-sided P-value less than 0.050 was considered statistically significant. Pairwise comparison was conducted to compare any two-attack rates in different quarters and aetiologies by the partitions of the χ2 method and Z-test for comparing column proportions. The basic idea of partitions of the χ2 method is to divide a crosstable into smaller four-cell tables for multiple comparisons, thereby increasing the probability of making a type I error. Therefore, adjusting the test level(α) to account for this increased probability of error is recommended. The adjusted test level (α’) is calculated as follows: α’ = (2α)/[k(k-1) +1], where k refers to the number of sample rates. When the attack rate of different quarters or aetiologies were compared pairwise, α’ was reduced to 0.008 and 0.003, respectively. Distribution fitting was performed using the MATLAB R2023a software. All figures and tables were designed using Origin version 2022 and Microsoft Word software version 2019.
Results
General characteristics
From 2012 to 2022, Wenzhou reported 198 outbreaks to the FDOSS, averaging 18 outbreaks annually. As shown in Table 1, these outbreaks included 2,216 cases, averaging 11.2 cases per outbreak, 208 hospitalisations, and eight deaths over the 11 years. In addition, the morbidity from foodborne outbreaks ranged from 1.0 to 3.6 cases per 100,000 people.
Table 1. Number of outbreaks, cases, hospitalisations and deaths by year, Wenzhou, China, 2012–2022

Time distribution
Foodborne outbreaks showed a seasonal pattern, with the highest number of outbreaks, cases, hospitalisations, and deaths occurring in the third quarter. Compared with the other three quarters, the third quarter accounted for 42.9%, 41.0%, 26.9%, and 87.5%, respectively (Table 2). Outbreaks occurred mainly between May and September, whereas November, January, and February had the fewest outbreaks (Figure 1). There was a significant difference in attack rates among the four annual quarters. According to α’ = 0.008 test level, differences in attack rate were significant in all quarter pairwise comparisons (p < 0.008) except between the first and fourth quarters (p = 0.028) and between the second and fourth quarters (p = 0.707). The pairwise comparison results were consistent with partitions of the χ2 method by Z-test for comparing column proportions.

Figure 1. Stacked histogram of the number of foodborne outbreaks by month in Wenzhou, 2012–2022
Table 2. Number of outbreaks, exposed, cases, hospitalisations and deaths of quarters, Wenzhou, China, 2012–2022
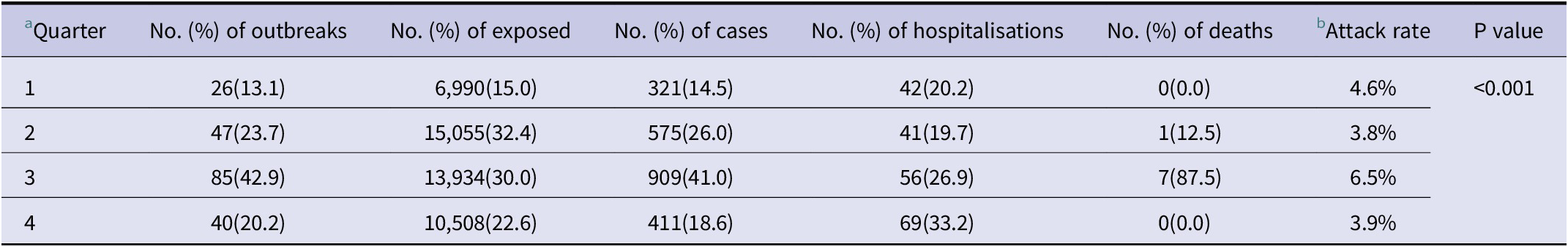
a Differences in attack rates among quarters were compared by Chi-Square test;
b Attack rates were calculated by the ratio of new cases to the number of people exposed.
Setting
Outbreaks were likely to occur in households (30.8%, 61), restaurants (29.6%, 60), and school canteens (18.7%, 37). Notably, school canteens (34.1%, 755) accounted for the highest number of cases, followed closely by restaurants (30.7%, 701) and households (13.1%, 290). Hospitalisations and deaths occurred mainly in households, accounting for 36.7% and 87.5%, respectively (Table 3).
Table 3. Number of outbreaks, exposed, cases, hospitalisations and deaths of settings, foods, and transmission pathways, Wenzhou, China, 2012–2022

Food and transmission pathway
More than 10 kinds of food have been associated with foodborne outbreaks. A total of 122 outbreaks had confirmed food sources, and 106 outbreaks were linked to a single food source, accounting for 61.6% and 53.5%, respectively (Table 3). Aquatic products had the highest number of outbreaks (17.7%, 35), followed by poisonous plants (10.6%, 21) and cereals (10.1%, 20) (Table 3). Meanwhile, the majority of cases were attributed to aquatic products (19.6%, 434), followed by meat and products (9.1%, 201) and cereals (8.4%, 185). The single food source that resulted in the most hospitalisations was cereals (25.5%, 53), followed by aquatic products (13.9%, 29) and poisonous plants (13.5%, 28). Notably, certain foods, such as liquor (100.0%), dairy (92.3%), mushrooms (91.0%), and flavourings (87.5%), exhibited higher morbidity rates. Mushrooms were the main cause of death from foodborne outbreaks (75.0%, 6).
Accidental ingestion was the primary mode of transmission (40, 20.2%), followed by multi-pathway and improper processing, which account for 12.1% and 11.1%, respectively. The leading contributing factors were personnel or equipment contamination (8,285, 17.8%) and multi-pathway (7,101, 15.3%). Notably, accidental ingestion was responsible for 69 hospitalisations (33.2%) and all eight deaths (Table 3).
Aetiology
The aetiologies were classified as microbiological, chemical, poisonous plants and animals, and mushroom toxins. Among these, microbiological aetiologies accounted for the highest number of outbreaks at 46.0%, followed by unconfirmed aetiologies, mushroom toxins, chemical aetiologies, and poisonous plants and animals accounting for 31.8%, 9.6%, 7.1%, 4.0%, and 1.5%, respectively (Table 4). Significant differences were observed in the attack rates of the six aetiologies (p < 0.001). According to α’ = 0.003 test level, differences in attack rates were significant in all pairwise comparisons of aetiology categories (p < 0.003) except between microbiological aetiologies and poisonous plants (p = 0.101) and between chemical aetiologies and poisonous animals (p = 0.168). The pairwise comparison results were consistent with partitions of the χ2 method by Z-test for comparing column proportions.
Table 4. Number of outbreaks, exposed, cases, hospitalisations and deaths of aetiologies, Wenzhou, China, 2012–2022

a Differences in attack rates among aetiologies were compared by Chi-Square test;
b Attack rates were calculated by the ratio of new cases to the number of people exposed.
Microbiological foodborne outbreaks were predominantly caused by bacteria with 82 (90.1%, 82/91) outbreaks; a smaller number were caused by viruses with 9 (9.9%, 9/91) outbreaks. The main bacterial species that led to foodborne outbreaks were V. parahaemolyticus, Salmonella ssp., and Staphylococcus aureus. In contrast, viral foodborne outbreaks were caused by norovirus (Figure 2A). In cases of chemical foodborne outbreaks, nitrite was the main cause, with rancidity, herbicides, and rat poison being the specific aetiologies (Figure 2B). Mushroom poisoning led to 19 outbreaks, 88 cases, and 22 hospitalisations. A total of 63 outbreaks, involving 512 cases and 27 hospitalisations, did not have an identified aetiology. Of the eight recorded deaths, six were caused by mushroom toxins, one by poisonous plants, and one by poisonous animals.

Figure 2. Number of outbreaks of microbiological aetiologies (A) and chemical aetiologies (B) distribution of foodborne outbreaks in Wenzhou, 2012–202
Incubation period
The incubation period of foodborne outbreaks was analysed and depended mainly on the aetiology and severity of poisoning (Table 5). For instance, V. parahaemolyticus has an incubation period of 2 to 43 h; Salmonella ssp. has a median of 15 h and 33 min, with a maximum of 669 h; symptoms caused by Bacillus cereus usually develop within 2 to 22 h; whereas FBD caused by norovirus develops within 4 to 70 h. Certain aetiologies, such as rancidity, nitrite, herbicides, poisonous animals, and plants, can cause symptoms to develop within a few minutes to a few hours after ingestion of food. Additionally, symptoms of mushroom poisoning can occur in as little as 30 min, as long as 24 h, or even days.
Table 5. The incubation period of cases of foodborne diseases

Gamm, Weibull, and Lognormal distribution were used to fit the probability distribution of the incubation period of confirmed cases, with the optimal distribution model being the one with the maximum R-square and the minimum AIC values (Figure 3). The results showed that the incubation period of confirmed cases was best fitted with a Lognormal distribution (R-square 0.998), which was superior to the Weibull (R-square 0.978) and Gamma (R-square 0.902) distributions. Furthermore, comparing the AIC values of the three models, the Lognormal distribution model had the best goodness of fit.

Figure 3. Fitting probability distribution of the incubation period (days) of confirmed cases
Food and aetiology
Our analysis demonstrated that the aetiologies of the outbreaks varied depending on the type of food consumed (Figure 4A). The foods most often responsible for microbiological outbreaks are aquatic products (27.5%, 25/91). Chemical foodborne outbreaks are caused by cereals (42.9%, 6/14), flavouring (14.3%, 2/14), vegetables (7.1%, 1/14), and beans (7.1%, 1/14), mainly involving nitrite poisoning. Outbreaks from poisonous animals have been attributed to aquatic products (100.0%, 3/3) contaminated with tetrodotoxins. Mushroom toxin outbreaks are mostly caused by poisonous mushrooms (100.0%, 19/19). At the same time, cereals (12.5%, 1/8), beans (25.0%, 2/8), liquor (25.0%, 2/8), vegetables (12.5%, 1/8), and poisonous plants (25.0%, 2/8) were the foods responsible for outbreaks caused by poisonous plants. Moreover, certain foods exhibited a common aetiology (Figure 4B). For example, outbreaks from dairy and beverages are both caused by microbiological aetiologies. In contrast, outbreaks from liquor are caused by poisonous plants, and those from flavourings are caused by chemical aetiologies. Certainly, certain foods can cause outbreaks of various aetiologies. The aetiologies of outbreaks caused by cereal consumption include microbiological (40.0%, 8/20) and chemical (30.0%, 6/20) aetiologies, and poisonous plants (5.0%, 1/20).

Figure 4. Aetiology-food (A), food-aetiology (B) distribution of foodborne outbreaks in Wenzhou, 2012–2022
Discussion
The findings of the present study provide novel and valuable information for identifying aetiologies, a fundamental objective in outbreak investigations. In this study, we investigated 198 reported foodborne outbreaks that occurred from 2012 to 2022 in Wenzhou of China. Survey data demonstrated that the rates of foodborne outbreaks were significantly higher in the third annual quarter than in the other three quarters. As for the setting, the outbreaks were mainly located in households and restaurants. Aquatic products caused the most outbreaks. Microbiological aetiologies were the main aetiologies of foodborne outbreaks, with bacteria being the most common pathogens.
For foodborne outbreaks, effective identification of the relationships among the crucial factors contributing to outbreaks can help guide appropriate measures to prevent future outbreaks, as well as help contain and reduce economic losses during outbreaks. Therefore, the necessity to analyse the epidemiological characteristics of foodborne outbreaks has been recognised for years [Reference Wu13].
The number of outbreaks remained steady between 2012 and 2014, followed by a substantial increase in 2015, after which there was a slight fluctuation. This surge in cases might be attributed to the gradual improvement of the FDSRS and FDOSS and the increased awareness among reporters from hospitals and units where suspected foodborne outbreaks occurred [Reference Liu14]. In 2015, the Food Safety Law was revised to improve the supervision of outbreak investigations and reduce under-reporting and cover-ups [Reference Zhang, Chen and Wu15]. Complete coverage of the FDSRS and FDOSS has improved the sensitivity and timeliness of reporting related to FBD, enabling early detection of potential food safety risk and timely measures to prevent further exposure and infection.
The surveillance data revealed a seasonal pattern of foodborne outbreaks in Wenzhou, with a higher occurrence during the third quarter, consistent with previous reports [Reference Li16]. The hot and wet conditions in Wenzhou during the third quarter create favourable conditions for the proliferation of bacteria, fungi, and plants, thereby increasing the likelihood of exposure to various aetiologies. Furthermore, households and restaurants were particularly vulnerable to foodborne outbreaks, which is consistent with previous research findings [Reference Li4]. The summer vacation coincided with the third quarter, leading to an increase in the frequency of children and parents dining in households and restaurants instead of school and staff canteens. Consequently, focusing on preventing foodborne outbreaks during the third quarter is crucial, particularly regarding microbiological aetiologies, mushroom toxins, and poisonous plants [Reference Wang17].
More than one-third of the foods responsible for the outbreak were not identified. This complexity is because people may be exposed to various mixed foods, or cross-contamination may occur in several foods [Reference Chen18] during outbreaks. Thus, it is often difficult to directly link specific foods to outbreaks. The main foods suspected of causing foodborne outbreaks vary greatly in different regions owing to dietary habits and geographical conditions. A survey of foodborne outbreaks in China found that poisonous mushrooms were the main food source [Reference Li19]. However, in Wenzhou, poisonous mushrooms caused only a small proportion of outbreaks, had the highest fatality rate, and were more commonly implicated in households. Foodborne outbreaks are primarily caused by aquatic products, which often carry V. parahaemolyticus.
Food can cause diseases through various contamination pathways and improperly cooked foods that carry pathogens. Among the contamination pathways, accidental ingestion due to the poor ability to distinguish poisonous food, such as mushrooms, and the difficulty distinguishing nitrite from salt accounts for the largest number of foodborne outbreaks. Moreover, a lack of hygiene awareness leads to contamination from personnel or equipment, and improper processing or cooking significantly contributes to foodborne outbreaks. Research has shown that food contamination by ill or infectious food workers contributes to approximately 40.0% of outbreaks with identified contamination routes [Reference Moritz20]. More attention should be paid to multi-pathway contamination, which is attributed to food storage and processing complexity. Consequently, raising awareness about toxic foods among residents and improving hygiene habits among food processors should be prioritised to cut off transmission pathways and prevent foodborne outbreaks [Reference Aquino21].
Surveillance results showed that microbiological aetiologies resulted in nearly half of foodborne outbreaks occurring widely in various settings [Reference He and Shi22]. Bacterial food poisoning is a common cause of outbreaks, with V. parahaemolyticus being the leading bacterium, followed by Salmonella ssp. and S. aureus. V. parahaemolyticus is generally found in seawater and aquatic products [Reference Li23], with a detection rate ranging from 47.5% to 66.5% in the coastal waters of East China and an average bacterial colonisation rate of 48.0% in aquatic products. During the third quarter, this rate exceeded 90.0%, confirming the high attack rate of foodborne outbreaks during this period. The coastal location and relatively developed aquaculture industry in Wenzhou are directly linked to the prevalence of V. parahaemolyticus.
Conversely, research conducted in Europe has indicated that S. Enteritidis remains the most frequently reported pathogen in foodborne outbreaks [24], which may be attributed to its relatively underdeveloped marine industry and low production of aquatic products [Reference Lofstedt, de Roos and Fernandes25]. This disparity may be explained by differences in eating habits and the development of the marine industry across regions. Given the significant association between V. parahaemolyticus, aquatic products, and Wenzhou, clear guidelines for health education should be developed to prevent foodborne outbreaks caused by this pathogen. These guidelines should include recommendations to avoid consuming raw aquatic products, particularly during the third quarter, and to employ alternative sterilisation methods, such as vinegar pickling, before consuming raw aquatic products. Mushroom toxins are another important cause of foodborne outbreaks. There are many types of poisonous mushrooms, and a large proportion of them are similar in appearance to edible mushrooms, which can easily cause accidental ingestion [Reference Sun26]. In addition, pathogens and chemicals and the severity of poisoning can lead to different symptoms and incubation periods. Rapid determination of the incubation period and symptom profile can help guide the search for the aetiology and track the source of infection.
The liquor that causes food poisoning is medicinal wine, which is fermented at home and adulterated with herbal products containing aconitine, an extract of wolfsbane (genus Aconitum). Fifteen cases of poisoning, including five deaths, were reported at a hotel in Chongqing and were most likely caused by homemade unlabelled medicinal liquor containing extremely high concentrations of toxic Aconitum alkaloids [Reference Zhou27]. It is an aconitine component that causes food poisoning. Incidents of poisoning from homemade medicinal wines are common. To reduce the risks and chaos associated with producing homemade medicinal wine, relevant departments should strengthen their supervision and conduct popular science education for the general public.
It is worth mentioning that 63 outbreaks (31.8%) were found without a confirmed aetiology, likely due to the complexity of the outbreaks, difficulty in collecting suspected foods, and limitations in laboratory testing capacity. Regular training courses on foodborne outbreaks surveillance techniques, including the investigation of work processes, specification requirements, and technical points, should be conducted to improve the skills of investigators. Laboratories should enhance their ability to identify different aetiologies by prioritising the introduction of equipment and training personnel and broadening their detection range for Campylobacter, Listeria monocytogenes, and Aeromonas. In addition, simultaneous high-throughput detection of multi-pathogen is a direction for future development. The prevention and control of foodborne outbreaks is about improving the ability to inspect finished products for hazardous effects or presence and avoiding hazards. Hazard Analysis and Critical Control Point (HACCP) is a preventive approach to ensuring food safety, and its research and applications in China are increasing [Reference Radu28, Reference Shi29]. It is mostly applied in production and post-production processes to ensure that no contaminants are present to make finished products unsafe and to design measures to reduce the risks of contaminants to a safe level.
This study analysed the epidemiological characteristics of foodborne outbreaks, including population, seasonal, and setting distributions. It also identifies high-risk suspected foods and pathogens, providing valuable information for establishing a better foodborne outbreaks surveillance system. Targeted measures should be implemented to prevent and control foodborne outbreaks, such as health education for the general public, food safety training for catering staff, and enhancing professionalism among investigators. More detailed personnel training and health education programs are provided in the Supplementary Materials.
This study has several limitations. First, under-reporting cases and foodborne outbreaks, including sporadic and self-cured mild cases, is inevitable when relying on hospital data and active reporting through the FDOSS. To address under-reporting, an openly accessible Internet-based crowd-sourcing platform has been promoted in the United States and 89 other countries to supplement existing programs on FBD reporting [Reference Quade and Nsoesie30], reflecting our ongoing efforts focus. Second, although our surveillance system detected the outbreak, the investigation may not have been conducted smoothly, and the gathered information may have been incomplete because the patients ignored the instructions. Furthermore, the epidemiological characteristics of foodborne outbreaks vary depending on environmental factors, dietary habits, and other variables; thus, the conclusions drawn from our study may not be universally applicable to all outbreaks.
Conclusions
This analysis provides a comprehensive summary of the characteristics of foodborne outbreaks and analyses the distribution of high-risk foods and their aetiologies in Wenzhou since 2012. Foodborne outbreaks are mainly caused by microbiological aetiologies, particularly bacterial contamination. This emphasises that any stage of food production can contaminate food, leading to outbreaks. Therefore, effective prevention of foodborne outbreaks requires improved dissemination of food safety knowledge and implementation of HACCP principles. In addition, the government should focus on strengthening management and improving the FDSRS and FDOSS to enhance the detection, investigation, and prevention of foodborne outbreaks.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268824001626.
Data availability statement
The study of the data is available from the Foodborne Disease Outbreaks Surveillance System, and these data are not publicly available.
Acknowledgements
We thanked the members in all participating CDCs for the efforts of more than ten years.
Author contribution
Data curation: L.C., D.L.; Funding acquisition: L.C., D.L.; Investigation: L.C., L.W., Y.C., S.G., M.C., D.L.; Methodology: L.C., L.W., Y.C., S.G., M.C., D.L., L.Z., Y.L.; Software: L.C.; Project administration: L.W., Y.C., S.G., M.C., D.L., Y.S.; Conceptualization: S.G.; Resources: Y.S., L.Z., Y.L.; Supervision: Y.S.; Validation: Y.S.; Formal analysis: Q.C.; Visualization: Q.C.; Writing – review & editing: Q.C.
Competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical statement
The Wenzhou Centre for Disease Control and Prevention Institutional Review Board determined this study was exempt because it used available data without personal identifiers (approval no. WZCDCLLSC2023–002).
Funding
Subsidy Funds of Public Health Programs of China.












