Background
Breast cancer (BC) is one of the most common malignancy worldwide, only slightly inferior to lung cancer in 2018 (Bray et al., Reference Bray, Ferlay, Soerjomataram, Siegel, Torre and Jemal2018). Despite the great progress in early detection and systemic treatment, BC is still a serious threat to women's health (Harbeck & Gnant, Reference Harbeck and Gnant2017), and the possible risk factors remain important studied topic. The major risk factors for sporadic BC are linked to hormone exposure (Harbeck et al., Reference Harbeck, Penault-Llorca, Cortes, Gnant, Houssami, Poortmans and Cardoso2019). DNA damage may accumulate during the menstrual cycles with the imbalance of estrogen and progesterone. The estrogen receptor (ER) can interact directly with growth factor receptors to enhance gene expression related to cell proliferation and survival (Williams & Lin, Reference Williams and Lin2013). Estrogen receptor and progesterone receptor (PR) are used as routine pathological markers (Clark, McGuire, Hubay, Pearson, & Carter, Reference Clark, McGuire, Hubay, Pearson and Carter1983). In the St. Gallen surrogate subtype classification, ER, PR, human epidermal receptor 2 (HER2), and the proliferation marker Ki67 are combined to classify tumors into intrinsic subtypes for proper treatment, including Luminal A-like subtype, Luminal B-like subtype (HER2-positive or HER2-negative), HER2-enriched subtype, and triple negative BC (Goldhirsch et al., Reference Goldhirsch, Winer, Coates, Gelber, Piccart-Gebhart, Thurlimann and Panel2013).
A link between personality and BC has been hypothesized since ancient Greek when Galen noted that melancholic women were more susceptible to cancer (Butow et al., Reference Butow, Hiller, Price, Thackway, Kricker and Tennant2000), and the research on personality and BC dates back to the 1950s (Reznikoff, Reference Reznikoff1955). Personality refers to individual's relatively stable predispositions and patterns of thinking, feeling, and acting (Carver & Connor-Smith, Reference Carver and Connor-Smith2010). Researchers have made enormous progress on the Big Five trait taxonomy to characterize personality, producing an initial consensus that we can differentiate five replicable factors of personality as summarized by the broad concepts of extraversion, agreeableness, conscientiousness, neuroticism, and openness to experience (John, Naumann, & Soto, Reference John, Naumann, Soto, John, Robins and Pervin2008). Among these factors, neuroticism, extraversion, and conscientiousness are most concerned in the health psychology field so far (Friedman & Kern, Reference Friedman and Kern2014). Notably, neuroticism and extraversion have been studied with more frequency in relation to the cancer trajectory (Dahl, Reference Dahl2010). Neuroticism represents emotional instability, vulnerability to negative affect, and proneness to anxiety (Barlow, Ellard, Sauer-Zavala, Bullis, & Carl, Reference Barlow, Ellard, Sauer-Zavala, Bullis and Carl2014). Extraversion reflects the level of ease and enjoyment of social interactions (Carver & Connor-Smith, Reference Carver and Connor-Smith2010). Average heritability estimate is 39% for neuroticism and 42% for extraversion in a meta-analysis including 58 behavior genetic studies (Vukasovic & Bratko, Reference Vukasovic and Bratko2015).
Personality may impact BC directly by altering neuroendocrine and immune function or indirectly by affecting lifestyle such as cigarettes smoking, alcohol drinking, diet, exercise, etc. (Hilakivi-Clarke, Rowland, Clarke, & Lippman, Reference Hilakivi-Clarke, Rowland, Clarke and Lippman1994). Specifically, Antoni et al. have built a model about neuroticism and BC (Antoni et al., Reference Antoni, Lutgendorf, Cole, Dhabhar, Sephton, McDonald and Sood2006), and neuroticism shows duality to BC. On the one hand, persons with high level of neuroticism are more likely to smoke (Morissette, Tull, Gulliver, Kamholz, & Zimering, Reference Morissette, Tull, Gulliver, Kamholz and Zimering2007) and to become dependent on alcohol (Zilberman, Yadid, Efrati, Neumark, & Rassovsky, Reference Zilberman, Yadid, Efrati, Neumark and Rassovsky2018). Neuroticism strengthens the magnitude of physiological response to stressors (Norris, Larsen, & Cacioppo, Reference Norris, Larsen and Cacioppo2007), and is related to the disruption of circadian rhythms (Murray, Allen, Trinder, & Burgess, Reference Murray, Allen, Trinder and Burgess2002) and abnormalities of the immune system (Bouhuys, Flentge, Oldehinkel, & van den Berg, Reference Bouhuys, Flentge, Oldehinkel and van den Berg2004). That is, neuroticism may facilitate the chronic overactivation of autonomic nervous system and disturbs endocrine and immune function, in turn leading to the initiation of BC (Friedman & Kern, Reference Friedman and Kern2014). On the other hand, appropriate neuroticism helps individuals be concerned about their health thus to live healthier (Turiano, Mroczek, Moynihan, & Chapman, Reference Turiano, Mroczek, Moynihan and Chapman2013). However, studies of the relationship between personality and BC have yielded conflicting results. Many studies reported no association between personality and BC (Jokela et al., Reference Jokela, Batty, Hintsa, Elovainio, Hakulinen and Kivimaki2014; Lillberg, Verkasalo, Kaprio, Helenius, & Koskenvuo, Reference Lillberg, Verkasalo, Kaprio, Helenius and Koskenvuo2002; Minami et al., Reference Minami, Hosokawa, Nakaya, Sugawara, Nishino, Kakugawa and Tsuji2015; Nakaya et al., Reference Nakaya, Bidstrup, Saito-Nakaya, Frederiksen, Koskenvuo, Pukkala and Johansen2010). But several studies found statistically significant results. For instance, a cohort study found strong positive association between neuroticism and the survival of BC patients with the hazard ratios (HRs) of 2.3 (Nakaya et al., Reference Nakaya, Hansen, Schapiro, Eplov, Saito-Nakaya, Uchitomi and Johansen2006). A recent cohort study found the relation of ‘type 1 personality’ with the decreased risk of BC (Lemogne et al., Reference Lemogne, Consoli, Geoffroy-Perez, Coeuret-Pellicer, Nabi, Melchior and Cordier2013). This personality type, characterized by suppressed emotional expression in the context of interpersonal relationships, correlates positively with neuroticism and inversely with extraversion (Heilbrun & Friedberg, Reference Heilbrun and Friedberg1988).
Given the confounding factors and the inconformity among studies on personality and BC, a novel research methodology is needed. Mendelian randomization (MR) is a genetic epidemiological approach in recent years that enables us to assess causal effects in observational datasets by exploiting germline genetic instrumental variants as unbiased proxies for exposure of interest (Lawlor, Harbord, Sterne, Timpson, & Smith, Reference Lawlor, Harbord, Sterne, Timpson and Smith2008). There are three key underlying assumptions: Assumption 1, SNPs as instrumental variants are robustly related to exposure; Assumption 2, SNPs are not associated with confound factors; Assumption 3, SNPs influence outcome via exposure only. Horizontal pleiotropy occurs when the last two assumptions are not met (Verbanck, Chen, Neale, & Do, Reference Verbanck, Chen, Neale and Do2018). Only when the underlying assumptions of MR are fulfilled can we make an inference about causal direction of association between personality and BC (Fig. 1). Since germline genetic variants of personality are randomly assorted at meiosis, MR analysis can be less likely to be confounded by environmental factors. Therefore, MR can be thought of as analogous to a randomized controlled trail (Little, Reference Little2018). Nevertheless, there is currently no MR analysis of personality and BC as far as we know.
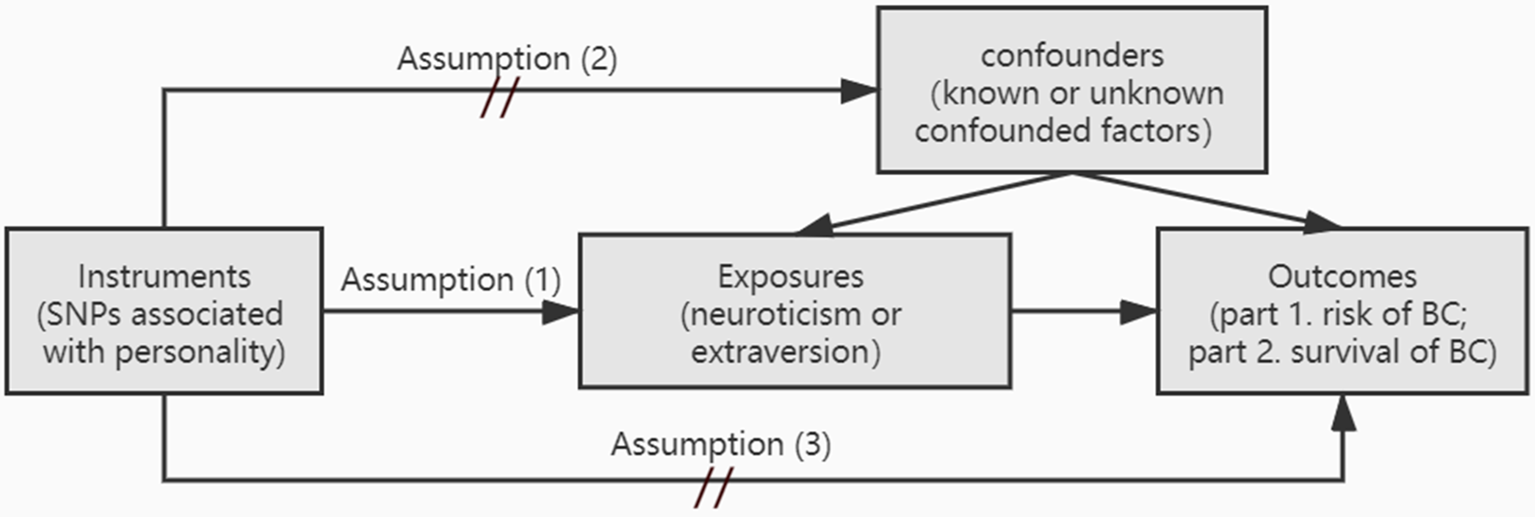
Fig. 1. Schematic representation of this MR analysis. Assumption (1): the instrumental variants are robustly related to exposure; Assumption (2): instrumental variants are not associated with confound factors; Assumption (3): instrumental variants influence outcome via exposure only; BC, breast cancer.
Here, we investigate whether personality is causally associated with the risk and survival of BC, as a whole or individual molecular subtypes, via a two-sample MR analysis.
Methods
Study design
This MR analysis consists of two parts (Fig. 1). Firstly, we test the association between personality and BC risk. Secondly, we investigate the association between personality and the survival of BC. There are three assumptions that need to be fulfilled: Assumption 1, the instrumental variants are robustly related to exposure; Assumption 2, instrumental variants are not associated with confound factors; Assumption 3, instrumental variants influence outcome via exposure only.
Genetic association with breast cancer
Genetic association with the risk and survival of overall and individual subtype BC was obtained from the Breast Cancer Association Consortium (BCAC). Summary statistical results from iSelect genotyping Collaborative Oncological Gene-Environment Study (iCOGS), OncoArray platform, and combined meta-analysis were provided by Michailidou et al., which included 122 977 cases with BC and 105 974 controls (Michailidou et al., Reference Michailidou, Lindstrom, Dennis, Beesley, Hui, Kar and Easton2017), and Escala-Garcia et al., which contained 96 661 women with BC and 7697 BC-specific deaths. (Escala-Garcia et al., Reference Escala-Garcia, Guo, Dork, Canisius, Keeman, Dennis and Schmidt2019). These results are for women of European ancestry only.
Selection of instrumental variants
We extracted 116 instrumental variables of neuroticism from a large-scale Genome-Wide Association Studies (GWAS) analysis (Luciano et al., Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali and Deary2018), which includes over 398 000 European ancestry individuals. As extraversion was less studied in GWAS, five variants of extraversion were extracted from Genetic of Personality Consortium (GPC) and 23andMe (Lo et al., Reference Lo, Hinds, Tung, Franz, Fan, Wang and Chen2017), which consists of over 80 000 European ancestry individuals. For SNPs unavailable in the BC dataset, proxies in linkage disequilibrium at r 2 > 0.8 were identified using SNIPA (Arnold, Raffler, Pfeufer, Suhre, & Kastenmuller, Reference Arnold, Raffler, Pfeufer, Suhre and Kastenmuller2015). Four SNPs of neuroticism were excluded from analysis since these variants as well as their proxies were not available in the dataset of outcomes. Three other SNPs of neuroticism were also excluded as they overlapped in BC (p < 5 × 10−8). The complete list of instruments is summarized in online Supplementary Table S1.
Statistical analysis
Two-ample MR analysis was performed to verify the potential causal link of personality to BC risk and survival (Hemani et al., Reference Hemani, Zheng, Elsworth, Wade, Haberland, Baird and Haycock2018). We used inverse-variance-weighted (IVW) multiplicative random-effects model to generate effect estimates as the major result (Burgess, Dudbridge, & Thompson, Reference Burgess, Dudbridge and Thompson2016). MR-Egger regression (Bowden, Davey Smith, & Burgess, Reference Bowden, Davey Smith and Burgess2015), weighted median, MR-robust adjusted profile score (MR-RAPS) (Zhao, Wang, Hemani, Bowden, & Small, Reference Zhao, Wang, Hemani, Bowden and Small2020), and maximum likelihood were applied as complementary analysis. The MR-Egger method consists of three parts: (1) a test for horizontal pleiotropy, (2) a test for the causal effect, and (3) an estimate of the causal effect. Different from other methods, MR-Egger considers the existence of horizontal pleiotropy and obtained the corrected estimates (Bowden et al., Reference Bowden, Davey Smith and Burgess2015). Funnel plot was also applied to test horizontal pleiotropy. Cochran's Q statistic was used to test the heterogeneity. MR-RAPS was used as correcting model while pleiotropy existed (Zhao et al., Reference Zhao, Wang, Hemani, Bowden and Small2020). Furthermore, the leave-one-out permutation analysis was used to verify whether the associations were provided by any individual SNP. MR analyses mentioned above were performed by using the TwoSampleMR R packages (Hemani et al., Reference Hemani, Zheng, Elsworth, Wade, Haberland, Baird and Haycock2018). Statistical significance threshold of p < 0.05 was used for these analyses. Power calculation was performed base on the mRnd website (https://shiny.cnsgenomics.com/mRnd/) (Brion, Shakhbazov, & Visscher, Reference Brion, Shakhbazov and Visscher2013).
Results
The associations between instrumental variants and all outcomes are summarized in online Supplementary Tables S3 and S4, and complete MR analyses were displayed in online Supplementary Table S5. As sensitivity analysis, leave-one-out permutation analyses were presented in online Supplementary Figs S1 and S2. Funnel plots were presented in online Supplementary Figs S3 and S4.
Instrumental variants
One hundred and sixteen independent SNPs were extracted from a large-scale GWAS study, which account for 10.8% variance of neuroticism (Luciano et al., Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali and Deary2018). Neuroticism was measured by the total score of the 12-item Eysenck Personality Questionnaire-Revised Short Form. Among these variants, four SNPs (rs1892984, rs7270023, rs1275411, rs5346666) were not available in the dataset of BC; three SNPs (rs7780406, rs2532386, rs199534) were significantly associated with BC. Therefore, 109 SNPs were utilized as instruments of neuroticism in MR analysis (online Supplementary Table S1). Five independent SNPs of extraversion were obtained and utilized from a GWAS of GPC and 23andMe (Lo et al., Reference Lo, Hinds, Tung, Franz, Fan, Wang and Chen2017).
Causality between personality and risk of breast cancer
The association between neuroticism and overall BC risk is statistically significant via IVW method [odds ratio (OR) 1.06; 95% confidence interval (CI) 1.01–1.11; p = 0.015]. The same result arises in weighted median (OR 1.08; 95% CI 1.02–1.14; p = 0.008), Maximum likelihood (OR 1.06; 95% CI 1.02–1.10; p = 0.002), and MR-Egger methods (OR 1.58; 95% CI 1.16–2.14; p = 0.004). After correcting the horizontal pleiotropy, there was still a positive association between neuroticism and overall risk via MR-RAPS approach (OR 1.06; 95% CI 1.01–1.12; p = 0.016). Further analysis about subtypes shows that luminal A-like BC is notably affected by neuroticism (OR 1.09; 95% CI 1.03–1.16; p = 0.004). No evidence of horizontal pleiotropy was found in this analysis. Similar trends were obtained via methods of weighted median (OR 1.07; 95% CI 0.99–1.17), Maximum likelihood (OR 1.10; 95% CI 1.04–1.15; p = 0.0004), MR-Egger (OR 1.51; 95% CI 1.01–2.25; p = 0.045), and MR-RAPS (OR 1.10; 95% CI 1.03–1.17; p = 0.004). In contrast, neuroticism shows no relation to other subtypes of BC such as luminal B-like, luminal B-like HER2 negative, HER2-enriched, and triple negative (Fig. 2). On the other hand, we found no associations between extraversion and the risk of BC, no matter overall or any subtypes (Fig. 3). These results suggest that neuroticism may increase the risk of BC and especially luminal A-like subtype.

Fig. 2. Association between neuroticism and the risk of overall breast cancer and individual subtypes. OR, odds ratio per standard deviation of neuroticism level; CI, confidence interval; p value, p value of the causal estimates.

Fig. 3. Association between extraversion and the risk of overall breast cancer and individual subtypes. OR, odds ratio per standard deviation of neuroticism level; CI, confidence interval; p value, p value of the causal estimates.
Causality between personality and survival of breast cancer
There was no evidence that neuroticism or extraversion was associated with the survival of BC (Figs 4 and 5). The major results of MR analysis by using IVW method indicate that neuroticism was not associated with the survival of overall BC (OR 1.03; 95% CI 0.93–1.14), ER+ BC (OR 1.06; 95% CI 0.92–1.22), or ER– BC (OR 1.06; 95% CI 0.86–1.31). Similarly, extraversion was found unrelated to the survival of overall BC (OR 0.86; 95% CI 0.36–2.73), ER+ subtype (OR 0.995; 95% CI 0.38–2.59), or ER– subtype (OR 0.86; 95% CI 0.22–3.29). Consistent results were yielded via other methods applied (online Supplementary Table S5).
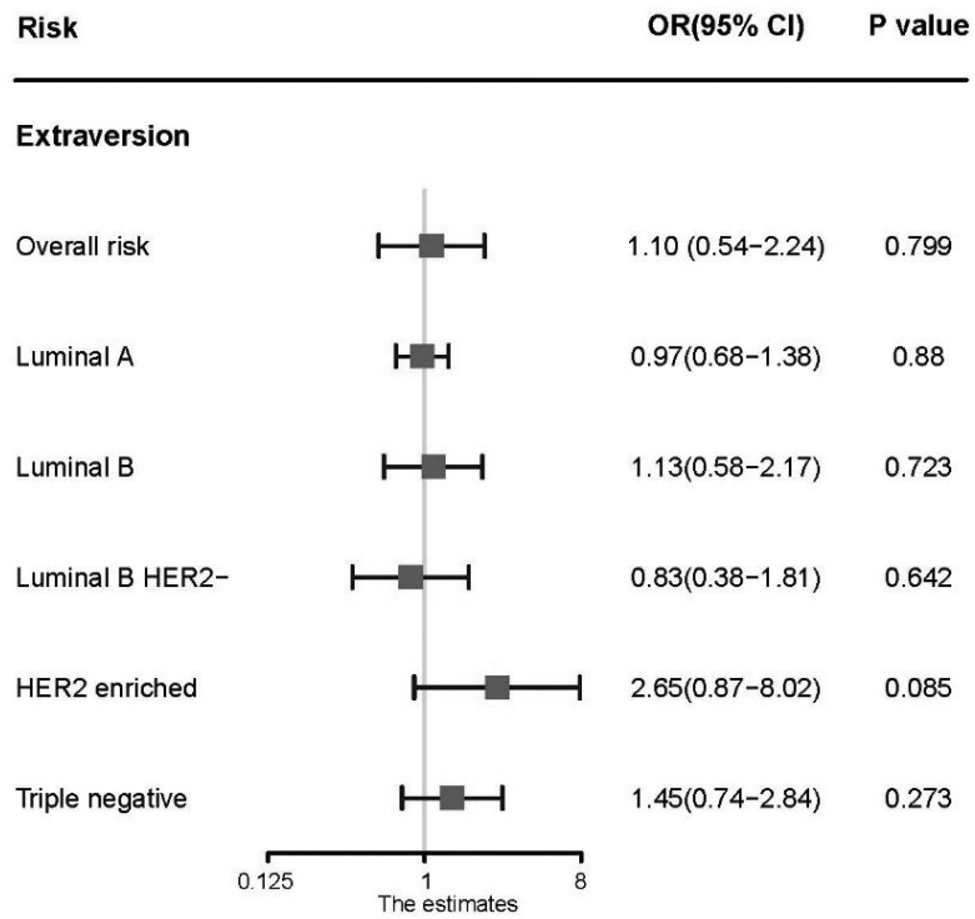
Fig. 4. Association between extraversion and the risk of overall breast cancer and individual subtypes. OR, odds ratio per standard deviation of extraversion level; CI, confidence interval; p value, p value of the causal estimates.

Fig. 5. Association between extraversion and the survival of overall breast cancer and individual subtypes. OR, odds ratio per standard deviation extraversion level; CI, confidence interval; p value, p value of the causal estimates.
Power calculation
Type-I error rate was set as 0.05 in performed power calculations. The selected instrumental variables explained 10.8% of the variation in neuroticism (Luciano et al., Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali and Deary2018). In the major results of MR analysis via IVW method, this study had 99% power to detect 6% greater odds of overall risk of BC per each standard deviation increases in neuroticism, and 99% power to detect 9% greater odds of luminal A BC risk per each standard deviation increases in neuroticism.
Discussion
Although many researches indicate that there is no significant association between personality and the risk of BC (Lillberg et al., Reference Lillberg, Verkasalo, Kaprio, Helenius and Koskenvuo2002; Minami et al., Reference Minami, Hosokawa, Nakaya, Sugawara, Nishino, Kakugawa and Tsuji2015; Nakaya et al., Reference Nakaya, Bidstrup, Saito-Nakaya, Frederiksen, Koskenvuo, Pukkala and Johansen2010), this study provides the evidence of modest but significant association between neuroticism and the risk of overall BC. Particularly, neuroticism affects luminal A BC at the molecular subtype level. Extraversion, however, shows no relation to the risk of BC. Neither of two composition of personality shows association with the survival of BC.
Although neuroticism has duality on BC, it increases risks of BC in general. In our analysis of neuroticism and the risk of overall BC, the results obtained by complementary methods are consistent with the major result. Among them, the result of MR-Egger is larger than others as it takes horizontal pleiotropy into analysis (Bowden et al., Reference Bowden, Del Greco, Minelli, Smith, Sheehan and Thompson2016). However, the result suggests that the association of neuroticism and BC risk is even larger after adjusting the horizontal pleiotropy. As horizontal pleiotropy is not significant in the analysis of neuroticism and the risk of luminal A-like subtype BC, the result of MR-Egger is more biased (Burgess & Thompson, Reference Burgess and Thompson2017). Nevertheless, the results of complementary analysis support the association between neuroticism and the risk of luminal A-like subtype BC. Previous prospective cohort studies about personality and the risk of BC usually adjusted inevitable confounding factors by statistical method (Lemogne et al., Reference Lemogne, Consoli, Geoffroy-Perez, Coeuret-Pellicer, Nabi, Melchior and Cordier2013; Lillberg et al., Reference Lillberg, Verkasalo, Kaprio, Helenius and Koskenvuo2002; Minami et al., Reference Minami, Hosokawa, Nakaya, Sugawara, Nishino, Kakugawa and Tsuji2015; Soler-Vila, Kasl, & Jones, Reference Soler-Vila, Kasl and Jones2003). These potential confounders include unhealthy lifestyle, life stress, education level, age at menarche, menopausal status, body mass index, and so on. Among these confounders, bio-behavioral factors such as life stress, psychological processes, and health behaviors influence tumor-related processes through neuroendocrine regulation of hormones (Antoni et al., Reference Antoni, Lutgendorf, Cole, Dhabhar, Sephton, McDonald and Sood2006). Neuroticism is the background factor for many of these confounders (Fig. 6) (Lahey, Reference Lahey2009). Extraversion is also related to lifestyle such as smoking, drinking, and exercising (Otonari et al., Reference Otonari, Nagano, Morita, Budhathoki, Tashiro, Toyomura and Takayanagi2012). Therefore, it is prone to generate new bias (weakening the effects of neuroticism, e.g.) in statistical adjustment of confound factors, which may account for different results in studies about personality and BC risk.
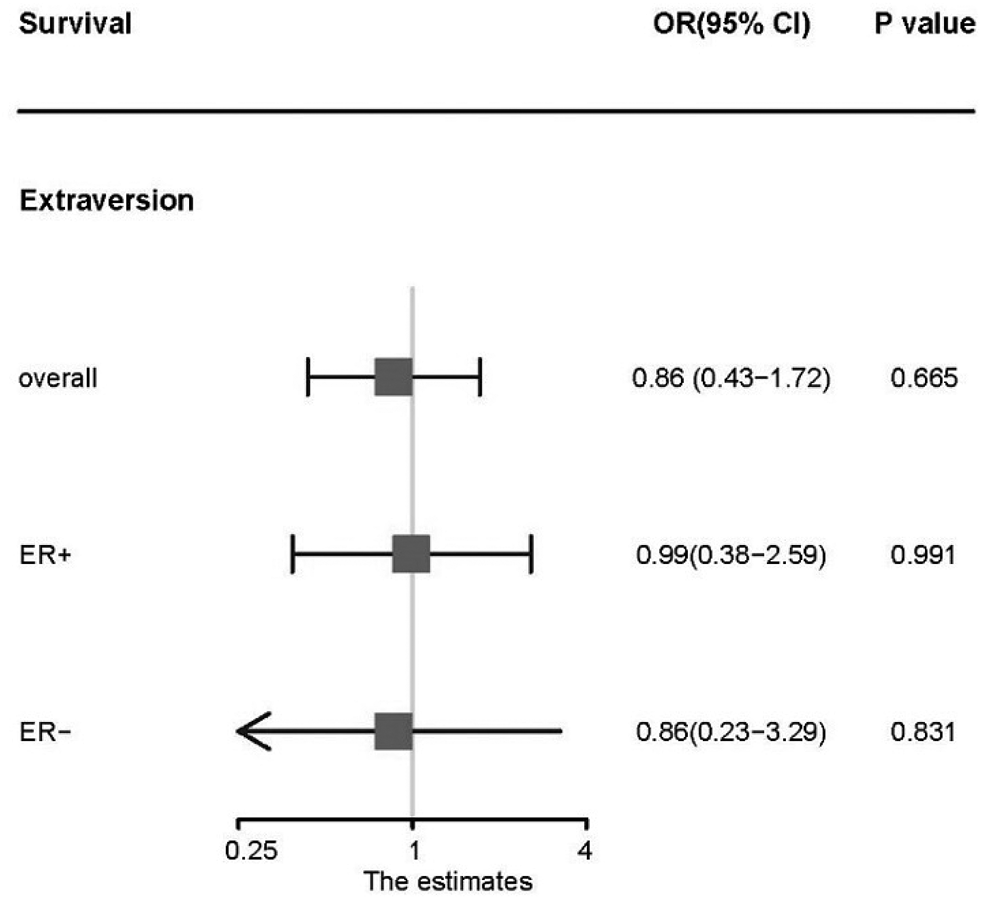
Fig. 6. Model for relation between neuroticism and breast cancer via neuroendocrine regulation [obtained from Antoni et al. (Reference Antoni, Lutgendorf, Cole, Dhabhar, Sephton, McDonald and Sood2006)].
Both neuroticism and extraversion may affect BC risk through lifestyle such as alcohol drinking and cigarettes smoking. However, neuroticism is associated with the heaviness of smoking and with the coping motives of drinking, while extraversion is associated with the initiation of smoking and with the enhancement motives of drinking (Hakulinen et al., Reference Hakulinen, Hintsanen, Munafo, Virtanen, Kivimaki, Batty and Jokela2015; Kuntsche, Knibbe, Gmel, & Engels, Reference Kuntsche, Knibbe, Gmel and Engels2006).
Moreover, neuroticism is closely related to mental disorders such as major depression disorder (MDD) and schizophrenia (Luciano et al., Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali and Deary2018). Neuroticism shares genetic loci with schizophrenia and increases the risk of schizophrenia, whereas extraversion reduced the risk (Smeland et al., Reference Smeland, Wang, Lo, Li, Frei, Witoelar and Andreassen2017; Van Os & Jones, Reference Van Os and Jones2001). Neuroticism shares up to two-thirds of genetic variance with MDD (Hettema, Neale, Myers, Prescott, & Kendler, Reference Hettema, Neale, Myers, Prescott and Kendler2006) and is strongly associated with the increased risk of MDD (Navrady et al., Reference Navrady, Ritchie, Chan, Kerr, Adams, Hawkins and McIntosh2017). As far as we know, schizophrenia is associated with a significantly increased risk of BC incidence in women (Zhuo & Triplett, Reference Zhuo and Triplett2018). MDD, manifested by persistent activation of hypothalamic-pituitary-adrenal axis, probably impairs the immune response and contributes to the initiation of BC (Soygur et al., Reference Soygur, Palaoglu, Akarsu, Cankurtaran, Ozalp, Turhan and Ayhan2007). Meanwhile, MDD and schizophrenia may increase the vulnerability for self-reported neuroticism scores (Navrady et al., Reference Navrady, Adams, Chan, Ritchie, McIntosh and Working2018). In a word, schizophrenia and MDD are important potential confounders which provide pleiotropy in the analysis, as they are closely related to neuroticism and are impossible to eliminate.
Significant association between neuroticism and luminal A subtype BC (strongly expressing ER and PR) rather than other subtypes indicates that neuroticism may affect the initiation of BC via neuroendocrine regulation (Fig. 6), while ER plays an important role in mediating the effects of endogenous hormones (Williams & Lin, Reference Williams and Lin2013). Although not significant, the impact of neuroticism on luminal B HER2-negative subtype BC (expressing ER and/or PR) shows consistent trend, which also suggests the potential role of neuroendocrine and ER. The result of horizontal pleiotropy test is not significant, which indicates that the influence of confounding factors is limited in this analysis.
As for the survival of BC, none of neuroticism and extraversion was influential. The result is consistent with most previous studies focusing on personality traits and the survival of BC (Soler-Vila et al., Reference Soler-Vila, Kasl and Jones2003; Watson, Homewood, Haviland, & Bliss, Reference Watson, Homewood, Haviland and Bliss2005). Personality having limited impact on survival may attribute to great progress of systemic treatment, which contains surgery, chemotherapy, endocrine therapy, targeted therapy, and psychological intervention.
This study using MR approach allowed for the estimation of causal effect of personality on BC with a large sample size and at high precision. Potential reverse causality and confound factors were prevented as much as possible by this method. The pleiotropic effects were detected and adjusted by the method of MR-Egger and MR-Raps (Zhao et al., Reference Zhao, Wang, Hemani, Bowden and Small2020).
Nevertheless, this study has several limits. SNPs were minimally excluded in order to maximize the strength of instruments, then several individual SNPs of neuroticism may be associated with confounding factors (schizophrenia, MDD, e.g.) thus weaken the robustness of the analytical results. The influences of these two mental disorders are difficult to separate while horizontal pleiotropy is significant in the analysis of neuroticism and overall risk. Therefore, we used different methods as supplementary analysis and sensitivity analysis. Besides, the analysis of extraversion was limited by scant SNPs. The results were deemed suggestive evidence of possible associations while considering the Bonferroni correction (p < 0.0027). As only European individuals were included in this study, further studies about different races are necessary for more conclusive results. Moreover, the specific mechanism of neuroticism affecting the initiation of BC needs further studies.
Conclusion
This is the first MR study about personality and BC. This MR analysis indicates that neuroticism on EPQ model is positively and modestly associated with the risk of BC and subtype luminal A BC. The result provides no support for the association between personality and the survival of BC. Assessment of neuroticism and psychological intervention may be helpful in early screening and prevention of BC.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721001562
Data
The summary statistics of GWAS datasets used in this study are available on request provided there is a clear statement of purpose.
Author contributions
Li Ying and Songzan Chen contributed equally to this work. Li Ying designed the study, contributed to the data analysis, and wrote the first draft. Songzan Chen contributed to the data analysis, and revision of the manuscript. Ling Li reviewed the design and contributed to data collection. The authors read and approved the final draft of the manuscript.
Financial support
No funds, grants, or other support was received.
Conflict of interest
None.
Ethical standards
All studies used in the current study were approved by relevant ethics committees. The protocol of BCAC was approved by each of the Ethics Committees of the participating institutions. All participants involved provided a written informed consent.









