In accordance with its pre-natal function, the ductus arteriosus shunts approximately 90% of blood from the pulmonary to the systemic circulation, and its patency is a vital component of fetal circulation. Reference Hamrick, Sallmon and Rose1 It is expected to close following birth due to changes in postpartum physiology. Closure of ductus arteriosus during the intrauterine period leads to severe pulmonary hypertension, which may advance to congestive heart failure, ultimately resulting in fetal demise. Reference Aslam and Christou2
The rupture of the valve chordae leading to in-utero tricuspid insufficiency due to intrauterine closure of ductus arteriosus is thought to be a lifesaving complication of pulmonary hypertension during the prenatal period. Reference Hornberger, Sahn, Kleinman, Copel and Reed3 This complication results in right ventricular off-loading that provides longevity since perfusion through the foramen ovale occurs. Cyanosis and exacerbation of congestive heart failure may be inevitable in the post-natal life of neonates possessing such pathology. Reference Sachdeva, Fiser, Morrow, Cava, Ghanayem and Jaquiss4
In this report, we present our management strategy in a newborn with congenital rupture of tricuspid chordae tendinea.
Case report
Since the manuscript describes a single case, ethics approval from the university was not obtained. An approval from review board of the associated institutions was obtained, and the paper was prepared afterwards. Patient’s family and first-grade relatives were informed about the protocols, procedures, risks and benefits, as well as possible use of the recorded materials in the hospital’s database system for academic purposes, and a signed consent was obtained from the parents.
A 3630-g female neonate born at 37 + 3 weeks of gestation who presented with respiratory distress and cyanosis was intubated and transferred from an external facility to our institution with suspicion of a CHD. The mother stated a history of a completely normal pregnancy and did not define neither COVID-19 infection nor the use of any medications or pain killers, except for folic acid and vitamin B12 supplementation. The neonate had an oxygen saturation of 75% with a 3/6 pansystolic murmur on physical examination. Despite increasing the fraction of oxygen to 100%, the saturation level did not exceed 90%. Prostaglandin E1 infusion of 0.1 mcg/kg/min was started. On echocardiography, the segmental anatomy was normal, and the ductus arteriosus was obliterated. An atrial septal defect was present with a right to left shunt. The right ventricle and the right and left atria were dilated. There was severe tricuspid insufficiency with a hyperechogenic tricuspid valve leaflet and ruptured chordae tendinea of the anterior leaflet prolapsing into the right atrium (Fig 1, Supplementary 1 and 2). Patency could not be achieved with a prostaglandin infusion. Therefore, the condition of the baby deteriorated rapidly and inotropes to overcome congestive heart failure were initiated. Emergency surgical treatment of the tricuspid insufficiency was planned after the retrieval of consent and informing the family about the risks and benefits of the surgery.
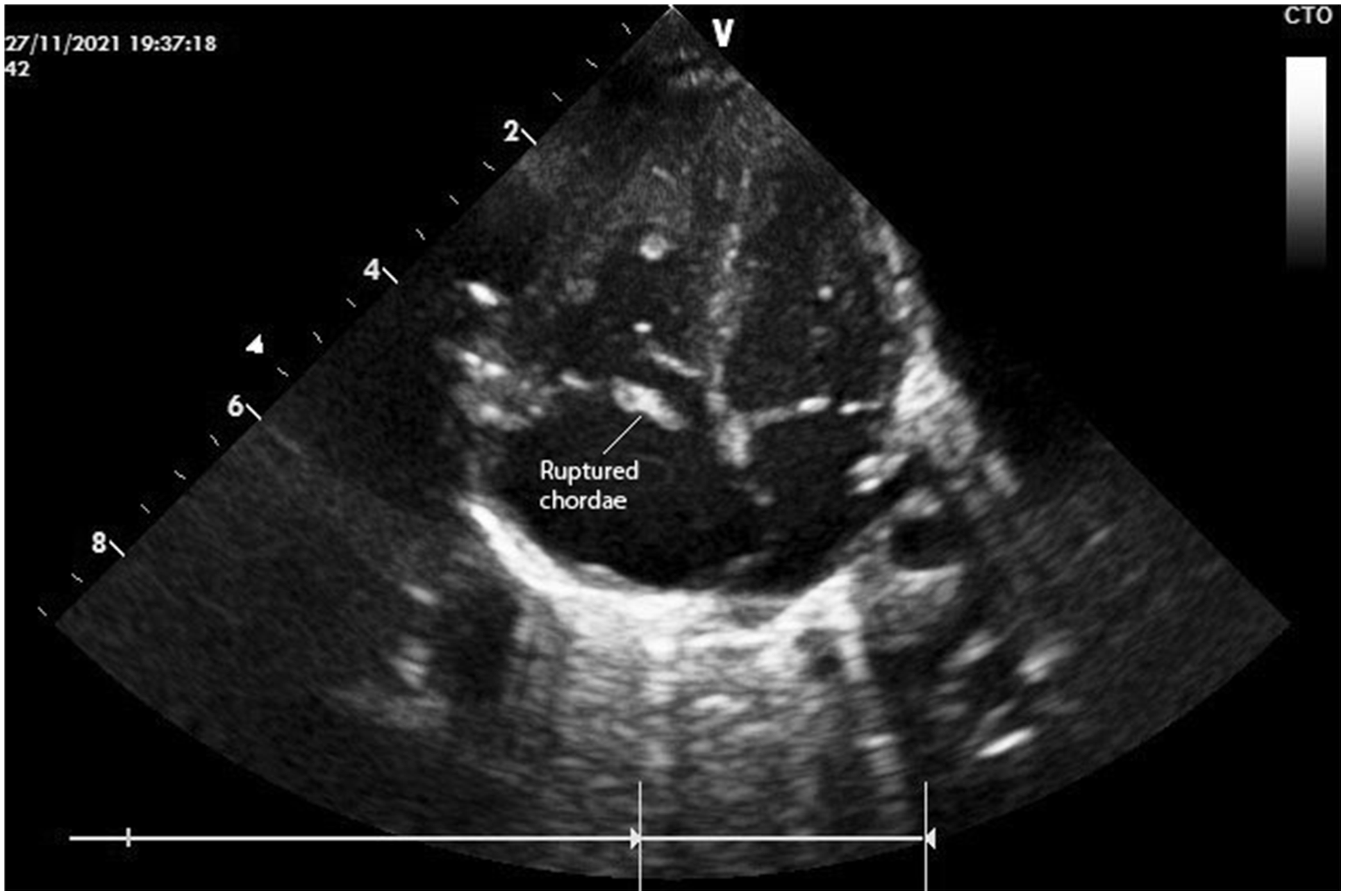
Figure 1. Preoperative echocardiography showing the flail leaflet and ruptured chordae.
The operation was performed on the second day of life through a midline sternotomy with an aortic and bicaval cannulation of the heart. Cardiac arrest was achieved with antegrade cardioplegia. A right atriotomy was performed parallel to the Waterston groove. On inspection, there was a secundum-type atrial septal defect and a ruptured chordae tendinea of the anterior leaflet (Fig 2). The ruptured chorda was reimplanted to the appropriate position, and the anterior leaflet was reinforced with an additional 5.0 PTFE (Gore-Tex; W. L. Gore & Associates, Flagstaff, AZ, USA) suture material (Fig 3). Both needles of the Teflon-reinforced PTFE suture were passed through the papillary muscle and then through the rough zone of the leaflet. Sutures were left untied but clipped. Initially, the length of the neo-chord was adjusted according to the reference point which was the adjacent chordae. The right ventricle is filled with saline and intraventricular pressure is increased. The dome or prolapse of the leaflet is adjusted with additional stretching or loosening clips until there is no prolapse and good tricuspid competence. Then the arms of the PTFE suture are tied and clips are removed. Saline test of the corrected valve revealed competent. The atrial septal defect was partially closed leaving a 2–3 mm functional defect which in return allowed for the off-loading of the right ventricle in case of a dysfunction. The operation was finalized. Perioperative transesophageal echocardiography showed trivial tricuspid insufficiency and bidirectional shunt through the atrial septal defect with good myocardial functions.
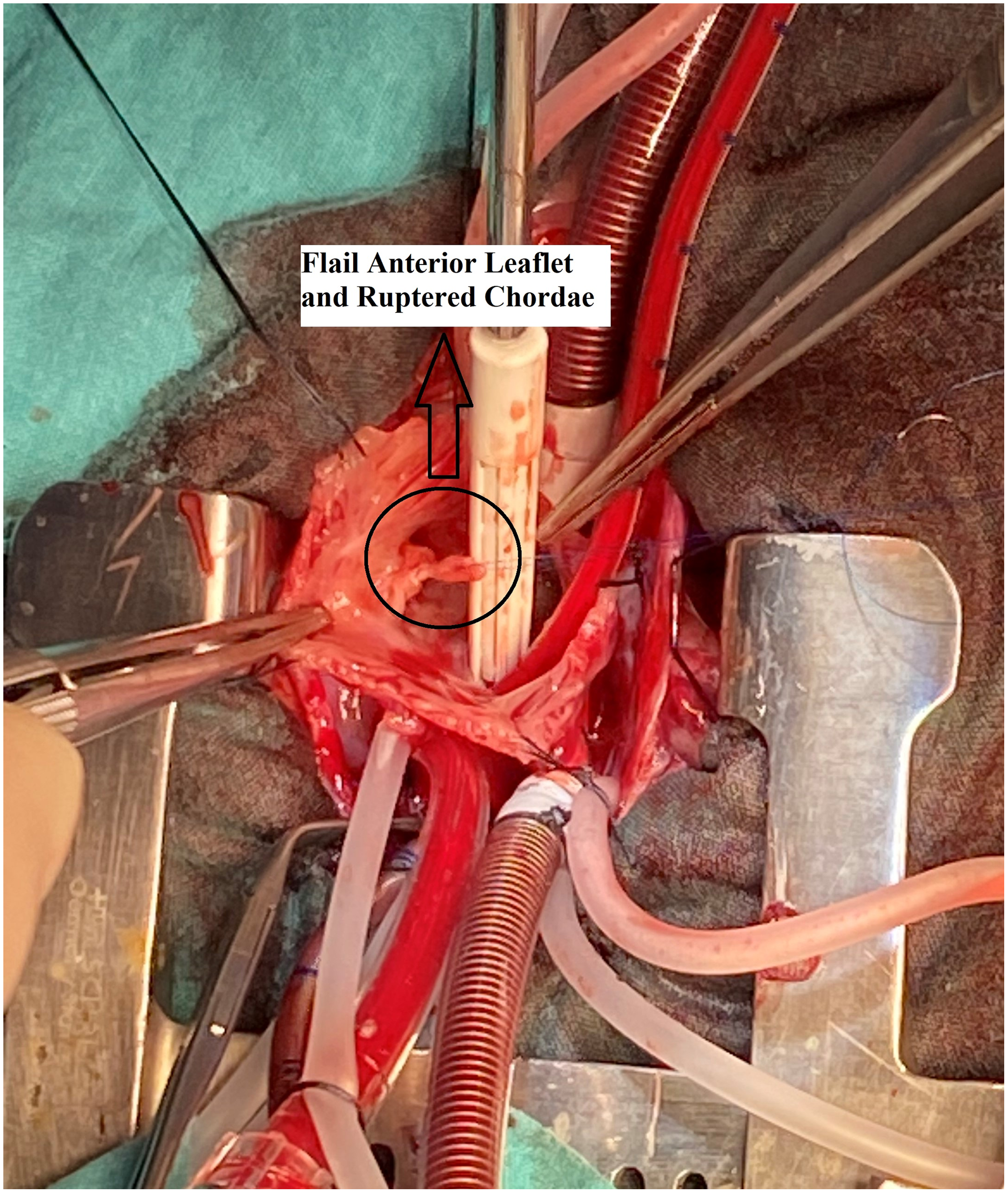
Figure 2. Perioperative view of the flail leaflet and ruptured chordae.
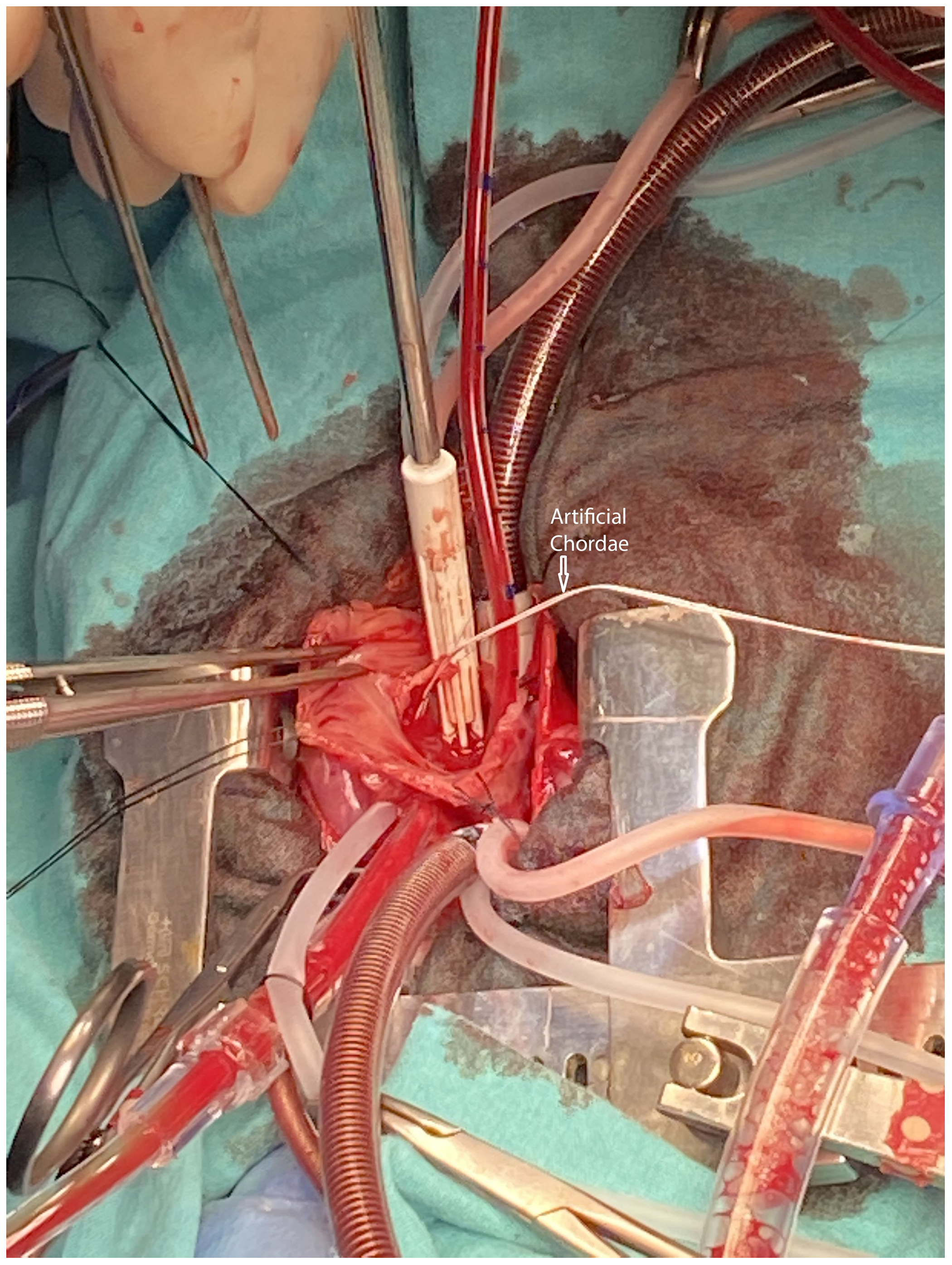
Figure 3. The pathology was treated with reimplantation of the ruptured chordae to its correct position and the anterior leaflet was further secured with a PTFE neo-chorda.
The patient was transferred to the ICU and received low-dose inotropic support (0.5 mcg/kg/min milrinone and 0.03 mcg/kg/min adrenaline) which were gradually decreased. She could be weaned off mechanical ventilation on the fourth day. The post-operative oxygen saturation was within normal limits (>95%). She was transferred to the neonatal ICU the next day and was discharged from the hospital in a week after performing routine assessments of infancy.
She has been followed actively; she is symptom free with a normal growth pattern. The echocardiography performed during follow-up, on the 5th month following surgery indicated a mild tricuspid insufficiency, insignificant left to right shunt through the atrial septal defect and normal myocardial functions.
Discussion
Myocardial ischaemia due to perinatal asphyxia, premature closure of the ductus arteriosus, congenital endocarditis, maternal auto-immune diseases, thromboembolism, a traumatic event during partum, and other congenital conditions are all pathologies that may lead to tricuspid valve chordae or papillary muscle rupture in a neonate. Reference Alkalay, Ferry, Pepkowitz, Chou, Oakes and Pomerance5 Even though the causes of antenatal closure of ductus arteriosus are not clear, the aetiologies that are thought to present more commonly include idiopathic closure and maternal exposure to non-steroidal anti-inflammatory agents. Reference Anagnostopoulos, Alphonso and Nölke6 The recent COVID-19 pandemic, which is associated with thrombosis, may also be accounted among the aetiologies thought to lead to tricuspid valve chordae or papillary muscle rupture in a neonate. The mother of the neonate did not state any medication use or a COVID-19 infection.
The intrauterine closure of ductus arteriosus may be a fatal condition if the right ventricle cannot offload consequently, leading to hydrops fetalis. The anterior leaflet of the tricuspid valve is located on the distal end of coronary perfusion and is susceptible to an ischemic insult. In addition, it is the most affected leaflet together with its sub-valvular apparatus due to defects in coronary perfusion as well as an increased right ventricular pressure. An additional right ventricular off-loading is necessary for the survival of the fetus when the ductus prematurely closes in utero. Intrauterine tricuspid insufficiency provides decompression of the right ventricle through the foramen ovale. Reference Pan, Lin and Chang7 We presumed the ductus of our case closed antenatally and survival of the fetus were feasible due to severe tricuspid insufficiency secondary to the ruptured chordae tendinea of the anterior leaflet. To stabilise the neonate, we attempted to re-open the ductus with prostaglandin E1 infusion after birth; however, it was not possible most probably due to an obliterated ductus arteriosus. Hence, the deteriorating condition of the baby secondary to the development of congestive heart failure despite inotrope support as well as continued cyanosis, resulted in a decision for early surgical treatment.
There are sparse reports in literature regarding survival of cases with intrauterine closure of the ductus arteriosus. Jun Yen Pan et al. presented a case of neonatal tricuspid regurgitation due to chordae rupture. They implanted a polytetrafluoroethylene artificial chorda over the unsupported margin of the anterior leaflet and successfully treated the neonate. Reference Pan, Lin and Chang7 Sachdeva R et al. also presented two cases with papillary muscle rupture of the tricuspid valve due to premature closure of ductus arteriosus. Due to critical cyanosis, one of the patients was operated following 12 days of extracorporeal membrane oxygenation support meanwhile the other patient was operated following 18 hours of support. Both patients were managed successfully. Reference Sachdeva, Fiser, Morrow, Cava, Ghanayem and Jaquiss4 Kaulitz et al. presented a case of tricuspid insufficiency due to a ruptured chorda of the anterior leaflet. They found that the ductus had an aneurysm with a constriction at the pulmonary site and thrombus formation in the remainder to the aortic site. Their successful surgical treatment was proceeded by postoperative nitric oxide support to decrease pulmonary pressure and facilitate extubation. Reference Kaulitz, Haen, Sieverding and Ziemer8 We operated on our case on the second day of life with techniques similar to the ones defined in literature, such as reimplantation of the ruptured chordae together with reinforcement of the flail anterior leaflet with a PTFE neo-chord. Postoperative course was uneventful and neither extracorporeal membrane oxygenation support nor nitric oxide was required.
In conclusion, congenital tricuspid insufficiency is an extremely rare condition. It may occur secondary to intrauterine closure of arterial duct. This pathology requires emergency treatment; otherwise, patients may die due to congestive heart failure. Surgery is the main option for correction of the disease in the current era and seems to be best performed with reimplantation of the chordae tendinea and additional securing of the leaflet with neo-chords.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1047951122001482
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Conflicts of interest
None.
Ethical standards
Manuscript is prepared in accordance with ethical standards.






