Following myocardial infarction the prevalence of major depressive disorder or elevated depressive symptoms is relatively high, at approximately 20%, Reference Thombs, Bass, Ford, Stewart, Tsilidis and Patel1 compared with around 5% in otherwise healthy people of comparable age. Reference Beekman, Copeland and Prince2 Elevated symptoms of depression, as measured with symptom questionnaires such as the Beck Depression Inventory (BDI), are present in around 30% of patients with myocardial infarction. Reference Thombs, Bass, Ford, Stewart, Tsilidis and Patel1 Post-infarction depression has been associated with a worse prognosis, and investigations into the strength of this association have been summarised in a number of meta-analyses, which showed that after myocardial infarction patients with depression were 1.59-2.71 times more likely to die early or have a new cardiovascular event than patients without depression. Reference Barth, Schumacher and Herrmann-Lingen3-Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge5 Such meta-analyses based on summary data, however, have serious limitations. By conducting an individual patient data (IPD) meta-analysis, a new statistical approach in this field, the main limitations can be overcome.
A major point of discussion in this field is whether depression is an independent risk factor for worsened cardiac outcomes, or whether its association with outcome is the result of non-causal mechanisms. Reference Frasure-Smith and Lesperance6 Most importantly, cardiac disease severity may confound the association between post-infarction depression and prognosis. Depression and disease severity - as measured by (for example) left ventricular ejection fraction (LVEF) or Killip class - are associated, and evidence suggests that people with more severe cardiac disease have a higher risk of depression. Reference Van Melle, de Jonge, Ormel, Crijns, van Veldhuisen and Honig7-Reference Freedland, Rich, Skala, Carney, Davila-Roman and Jaffe9 This may be the result of a psychological response to the disease and its consequences, as well as of physiological mechanisms involved in cardiac disease leading to symptoms of depression, such as elevated inflammation, Reference Raison, Capuron and Miller10 and changes in functioning of the autonomic nervous system or the hypothalamic-pituitary-adrenal (HPA) axis. Reference Evans, Charney, Lewis, Golden, Gorman and Krishnan11,Reference Carney, Freedland, Miller and Jaffe12 Evidently patients with more severe disease are also at higher risk of adverse cardiac outcomes, such as new cardiac events, readmittance to hospital and cardiac mortality. Similarly, other medical risk factors such as smoking and diabetes are likely to be associated with both disease severity and depression. Reference Golden, Lazo, Carnethon, Bertoni, Schreiner and Diez Roux13,Reference Anda, Williamson, Escobedo, Mast, Giovino and Remington14 Therefore, more severe disease and exposure to other risk factors could result in both a higher prevalence of depression as well as worsened cardiac prognosis, and thereby confound the association between depression and coronary artery disease outcomes.
Individual studies find conflicting results when the association between post-infarction depression and prognosis is adjusted for disease severity, some finding it to be attenuated, whereas others concluded the association remains unchanged. Previous systematic reviews and meta-analyses were only able to provide estimates of unadjusted associations, or very limited estimates of adjusted associations. This is due to the wide variability of adjustments in individual studies, making comparisons across studies impossible. Investigating the effects of adjustment is important, however, as variables related to cardiac disease severity, Reference Carney, Blumenthal, Catellier, Freedland, Berkman and Watkins15-Reference Parashar, Rumsfeld, Spertus, Reid, Wenger and Krumholz17 and other health-related variables, Reference Golden, Lazo, Carnethon, Bertoni, Schreiner and Diez Roux13,Reference Anda, Williamson, Escobedo, Mast, Giovino and Remington14,Reference Lee, Woodlief, Topol, Weaver, Betriu and Col18-Reference Denollet, Martens, Smith and Burg20 are prognostic factors for all-cause mortality and cardiovascular events, and are associated with depression.
The only way to investigate adequately the effects of cardiac disease severity and other medical risk factors on the association between post-infarction depression and prognosis is to combine data from individual studies into a single database. This has a number of advantages. First, with one large data-set all combined data can be analysed with the same techniques, whereas in summary data meta-analyses results are based on different statistical techniques, limiting their comparability. Second, the combined data-set offers the possibility of performing new analyses both within and across studies to investigate research questions that were not considered in the original studies. Reference Cooper and Patall21 Third, we can adjust consistently for the same variables across studies, providing us with a better estimate of their role in the association between depression and prognosis. Fourth, the combined database contains more raw patient data, which increases the statistical power, generalisability and reliability of the results, Reference Simmonds, Higgins, Stewart, Tierney, Clarke and Thompson22-Reference Van Walraven25 providing a more precise estimate of the association between post-infarction depression and prognosis, and of the effects of the individual variables. Reference Poppe, Doughty, Yu, Quintana, Moller and Klein24 Finally, time-to-event analyses can be performed, to utilise information on not only whether an event occurred, but also when it occurred, Reference Van Walraven25 which is one of the main advantages of this form of meta-analysis. Reference Stewart and Tierney26 The objective of our study, therefore, was to conduct an IPD meta-analysis that allowed for adjustment for a number of important disease severity variables and other health factors that are routinely collected in studies of depression following myocardial infarction.
Method
Studies included in our meta-analysis had been previously selected for two regular, summary data meta-analyses on post-infarction depression and cardiac prognosis. Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge5,Reference Van Melle, De Jonge, Spijkerman, Tijssen, Ormel and Van Veldhuisen27 A literature search was performed on 5 January 2011 to identify prognostic studies that investigated the association between post-infarction depression and cardiac prognosis since 1975. Depression treatment studies in which baseline depression scores and all-cause mortality or cardiovascular events outcomes were reported were also eligible. Relevant articles were selected from the electronic databases Medline (PubMed), EMBASE and PsycINFO without language restrictions. Search terms related to depression and myocardial infarction were used and customised to each database. Full search strings for each database are listed in online Appendix DS1. In addition to the database searches, major reviews and relevant articles were cross-referenced. Search alerts for the three databases mentioned above were activated to identify relevant studies published after 5 January 2011. All studies included in the summary data meta-analyses were eligible for inclusion in the IPD meta-analysis.
Selection process
The selection process has been described in detail elsewhere. Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge5,Reference Van Melle, De Jonge, Spijkerman, Tijssen, Ormel and Van Veldhuisen27 In summary, studies were selected by two independent raters according to the following criteria:
-
(a) patients had to be admitted to hospital for myocardial infarction;
-
(b) depression had to be determined within 3 months after the myocardial infarction using methods originally designed to assess depression (standard self-report questionnaires or standardised psychiatric interviews) and validated elsewhere;
-
(c) studies had to be prospective and assess cardiovascular prognosis in a patient group with depression compared with a control group without depression;
-
(d) outcome had to be all-cause mortality or cardiovascular events (the latter defined as either a non-fatal cardiac event or a composite of fatal and non-fatal cardiac events);
-
(e) the study had to be based on original data.
Authors of all the studies included in the summary data meta-analysis were contacted and invited to participate in the project. Considerable effort was put into finding and contacting authors and in obtaining all available databases. When corresponding authors could not be contacted at the address specified in the original articles, we searched the most recent articles and the internet for updated information on the authors' locations, and tried to contact other members of the research groups. Authors were asked to share their original data regarding demographic factors, depression, disease severity, comorbidities, medication use and outcomes. Data were checked for potential errors, and authors were contacted regarding questions related to the design of their study or the data-sets they provided.
Depression
Depression had to be measured using established self-report questionnaires or standardised structured diagnostic interviews. For the main analyses continuous scores on the self-report questionnaires were used. Dichotomous scores were used for descriptive purposes only, and were based on structured diagnostic interviews when available, and on standard cut-off scores (literature-based) on the self-report questionnaires when no interview was available. When more than one depression measurement instrument was used, standardised structured diagnostic interviews were preferred over self-report questionnaires in constructing dichotomous scores. When several self-report questionnaires were used in the same study, the one most frequently used by the other studies was selected. Across the studies a number of different self-report depression questionnaires were used, so total depression scores on these questionnaires were standardised to z-scores for analyses. This was done within each study. For some patients, depression questionnaire total scores and dichotomous scores were not available owing to missing item scores on the questionnaire. When no more than 25% of the depression items were missing, item scores were imputed with the mean of the available items for that patient, to calculate total scores and dichotomised scores.
Disease severity
To investigate the role of cardiac factors in the association between post-infarction depression and prognosis, LVEF, Killip class and history of myocardial infarction were used to quantify disease severity. These variables were selected because they are known predictors of outcome following infarction, Reference Lee, Woodlief, Topol, Weaver, Betriu and Col18,Reference Mueller, Cohen, Braunwald, Forman, Feit and Ross19,Reference Khot, Jia, Moliterno, Lincoff, Khot and Harrington28-Reference Thombs, Ziegelstein, Parakh, Stewart, Abbey and Grace31 and were available for a sufficient number of patients. These variables may predict both more symptoms of depression, Reference Van Melle, de Jonge, Ormel, Crijns, van Veldhuisen and Honig7 and worse cardiac outcomes. Reference Carney, Freedland, Miller and Jaffe12 The variable LVEF was dichotomised into low (<40%) and normal (⩾40%), as not all studies included continuous values. Killip class was dichotomised into no heart failure (class I) and heart failure (classes II, III and IV), as the four-category scores were not available in all studies. History of myocardial infarction was dichotomised into ‘yes’ or ‘no’.
Other risk factors
Several other health-related risk factors were expected to affect the association between post-infarction depression and prognosis. Of these, diabetes, smoking and body mass index (BMI) were included in the adjusted analyses, as data on these variables were collected for a large number of cases. Reference Golden, Lazo, Carnethon, Bertoni, Schreiner and Diez Roux13,Reference Anda, Williamson, Escobedo, Mast, Giovino and Remington14,Reference Lee, Woodlief, Topol, Weaver, Betriu and Col18,Reference Mueller, Cohen, Braunwald, Forman, Feit and Ross19,Reference Golden, Lazo, Carnethon, Bertoni, Schreiner and Diez Roux32
Age and gender
Age and gender were included in the analyses for the minimally adjusted comparison model. They may explain part of the association, as they are both related to the risk of depression and to physical health prognosis.
Outcome: all-cause mortality and cardiovascular events
The outcomes of all-cause mortality and new cardiovascular events were considered in the analyses. All-cause mortality includes cardiac mortality, and it was included because outcome data on all-cause mortality is generally more readily available than specific data on (cardiac) causes of mortality or morbidity. Cardiovascular events as defined most commonly by the original study authors were accepted, and could be either fatal events, non-fatal events or a combination of both. Cardiovascular events included, for example, new myocardial infarction, unstable angina and coronary artery bypass graft (CABG) surgery. All-cause mortality and cardiovascular events may overlap when studies included cardiac death in both definitions.
Study characteristics
For each study the following characteristics were summarised: year that the study was initiated, percentage of men in the sample, inclusion and exclusion criteria, mean age, depression measure, percentage of patients with depression, mean depression scores, duration of follow-up and number of outcome events. Ethical approval was obtained by the individual studies, for which participants signed informed consent forms. The medical ethics committee of the University Medical Centre Groningen stated that no additional informed consent was required for our study.
Statistical analysis
The main analyses were performed in Stata version 11 on Windows XP. All studies except one included continuous depression scores, and a number of studies in addition contained a binary measure of clinical diagnosis of depression. The one study that did not contain continuous depression scores was excluded from our analyses. Reference Rafanelli, Milaneschi, Roncuzzi and Pancaldi33 First, hazard ratios (HRs) were calculated using multilevel Cox proportional hazards regression analysis for the studies with time-to-event data. Second, odds ratios (ORs) were calculated using logistic regression analysis for all studies, including those with dichotomous outcome information only (event v. no event without time-to-event data).
Multilevel model
The individual studies were included as a separate level, resulting in a multilevel model in which the variable ‘study’ was included as a random intercept. Patient groups in the various studies were likely to differ in systematic ways, for example because of differences in selection criteria, study methods or cardiac care. Reference Cooper and Patall21 Observations of participants within studies were therefore unlikely to be fully independent. By incorporating a random effect for ‘study’ in IPD meta-analysis we accounted for the fact that outcome rates might vary across studies. The possibility of a random slope was also investigated, as the strength of the association between post-infarction depression and prognosis may vary significantly between studies. As this did not appear to be the case, random slopes were not included in the final models. In the Cox regression analyses, contrast coding of −0.5/0.5 was used for dichotomous variables, to ensure between-trial variances were equal between groups (e.g. male v. female). Reference Smith, Williamson and Marson34,Reference Smith, Williamson and Marson35 Potential bias due to standardising depression scores within each study, with the risk of overlooking potential differences in effect due to differences in depression severity and prevalence between studies, was investigated by adding a variable to the model that described the level of depression per study (‘percentage depressed’, based on depression questionnaire scores), as well as an interaction variable of the standardised depression scores and the ‘percentage depressed’ variable. As the analysis showed that this bias was virtually non-existent, we did not include these variables in the final models.
Bootstrapping
A bootstrapping procedure with 1000 replications was used for the analyses to increase the robustness of the confidence intervals, Reference Sauerbrei and Schumacher36 and to account for the fact that some of the depression z-scores were not distributed normally.
Model construction
The models were built as follows. First, a base model to which subsequent adjusted models could be compared was created by including age, gender and the depression z-scores as predictors of prognosis. As our primary interest was in the influence of individual variables on the association between post-infarction depression and prognosis, we then added each preselected variable separately to this base model in minimally adjusted analyses. Not all studies had data on each of these variables, and to be able to compare differences between the base and adjusted models, cases lacking data on the relevant variable were excluded from the relevant analyses in both models. Cardiac disease severity was represented by history of myocardial infarction, Killip class and LVEF. Diabetes, smoking and BMI were added as risk factors for poor prognosis. A variable was considered to explain a substantial portion of the variance if it changed the effect size (log HR) by 5% or more. Reference Whooley, de Jonge, Vittinghoff, Otte, Moos and Carney37 Variables that were also significant predictors of outcome (P=0.05) were considered to add substantially to the variance. As not all studies had time-to-event data, additional logistic regression analyses were performed and these models were built in the same way as for Cox regression analyses.
Second, we investigated the extent to which the association between post-infarction depression and prognosis was attenuated by adjusting for all of the risk factors. As not all studies had data on all variables, these multivariable analyses could only be performed with data from a limited number of studies. Nevertheless, this provides the best estimate of the extent to which post-infarction depression independently predicts cardiac outcomes.
Model assumptions
The proportional hazards assumption for Cox regression was tested, as well as the assumption of linearity in the association between post-infarction depression and prognosis. The model assumptions were met in most cases; where they were not, the effects of violation of the assumption were further investigated and determined to be minimal. Analyses were therefore run for these models in the same way as the other models, for the sake of clarity and interpretability of the results.
Patient characteristics
Patient characteristics were presented separately for the depressed and non-depressed patient groups. Differences in these characteristics between these two groups were assessed with independent samples t-tests for normally distributed continuous variables and Mann-Whitney U-tests for non-normally distributed continuous variables. Dichotomous and categorical variables were compared using Pearson's chi-squared test.
Effects of non-participation of eligible studies
To investigate whether there were any systematic differences (acquisition bias) between participating and non-participating studies that might affect the results of the meta-analysis, Reference Clarke and Stewart38 we compared results of included and excluded studies concerning strength of the association and study characteristics.
Results
A total of 6145 articles were identified through the literature search, cross-referencing and personal communication, of which 28 studies were ultimately included in the meta-analysis. Two additional studies were identified through search alerts and personal communication, Reference Hosseini, Yousefnejad, Tabiban, Nesarhoseyni, Bagheri and Kiasari39,Reference Van den Brink, van Melle, Honig, Schene, Crijns and Lambert40 resulting in a total of 30 eligible studies. The authors of 16 studies provided data for the IPD meta-analysis, resulting in a combined database of 10 175 patients (Fig. 1). Fourteen studies were not included: seven because the authors could not be contacted, five because data were not available and two because the authors were not interested in participating. An overview of participating studies is given in online Table DS1. Complete reports of study design and methodology of the individual studies are published elsewhere. Reference Denollet, Martens, Smith and Burg20,Reference Thombs, Ziegelstein, Parakh, Stewart, Abbey and Grace31,Reference Rafanelli, Milaneschi, Roncuzzi and Pancaldi33,Reference Hosseini, Yousefnejad, Tabiban, Nesarhoseyni, Bagheri and Kiasari39-Reference Doyle, Conroy, McGee and Delaney55 An overview of non-participating studies is given in online Table DS2.
Study characteristics
The 16 participating studies originated from nine different countries. The mean sample size was 615 participants per study (s.d. = 711, range 61-2889). Studies originated from 1985 to 2006. Mean patient age per study ranged from 56 years to 65 years (mean 61) and the proportion of male participants ranged from 33% to 85% (mean 72). All studies included patients with myocardial infarction based on standardised diagnostic criteria. Most of the exclusion criteria concerned life-threatening illnesses or psychiatric disorders other than depression, myocardial infarction due to a surgical procedure (e.g. CABG or valve replacement), or cognitive or communication difficulties.
Depression was measured with a self-report depression questionnaire, a structured clinical interview, or both. The self-report depression scales that were used included the Beck Depression Inventory versions BDI-1A, BDI-II and BDI - Fast Screen (BDI-FS), the depression subscale of the Hospital Anxiety and Depression Scale (HADS-D) and the Zung Self-rating Depression Scale (SDS). Structured clinical interviews included the Depression Interview and Structured Hamilton (DISH), the Composite International Diagnostic Interview (CIDI) and the Structured Clinical Interview for DSM Disorders (SCID). The percentage of participants with depression was lower when based on diagnostic interviews (11-15%) than when based on elevated symptoms on self-report questionnaires (17-69%). Follow-up time ranged from 350 days to 2428 days (1-6.7 years), with a mean of 1151 days (3.2 years).
Patient characteristics
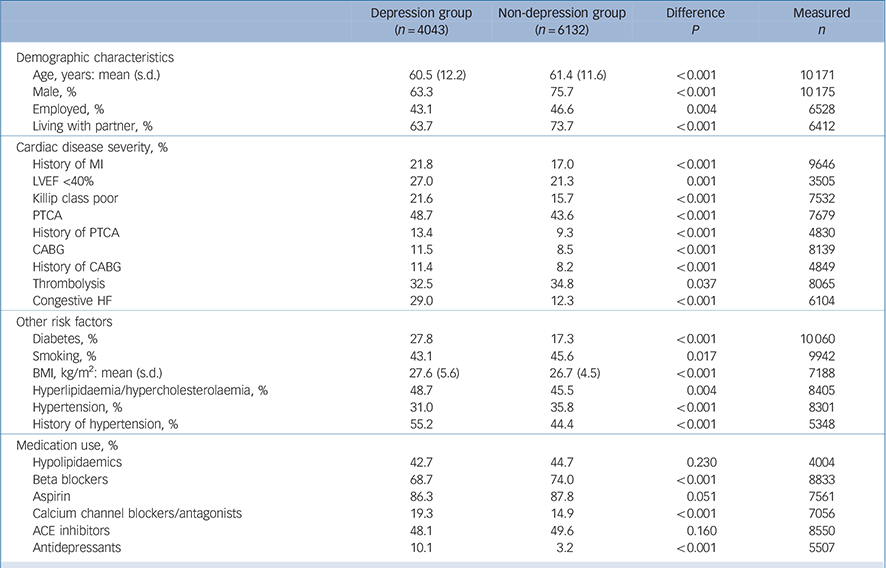
Individual patient data were combined for 10 175 persons with myocardial infarction; of these, 4043 (40%) had major depression or elevated symptoms of depression and 6132 (60%) were not depressed (note that some studies may have oversampled patients with depression, so these numbers may overrepresent depression percentages). Nineteen per cent of patients had a history of one or more myocardial infarctions prior to the index episode, 23% had low LVEF and 18% had a Killip class higher than I. Twenty-one per cent of those measured had comorbid diabetes, 45% were (ever) smokers and the mean BMI was 27 kg/m2 (Table 1).
Adjusted association between post-infarction depression and prognosis
Cox regression analyses
Base model (adjusted for age and gender). Figure 2 shows survival curves for the two outcomes all-cause mortality and cardiovascular events, with separate lines for the depressed and non-depressed patient groups, adjusted for age and gender. Rates of all-cause mortality were stable over time, and were consistently higher for the depressed group than for the non-depressed group. Rates of cardiovascular events were highest soon after the infarction and became relatively stable after about 1 year; again, rates were higher in the depressed group than in the non-depressed group.
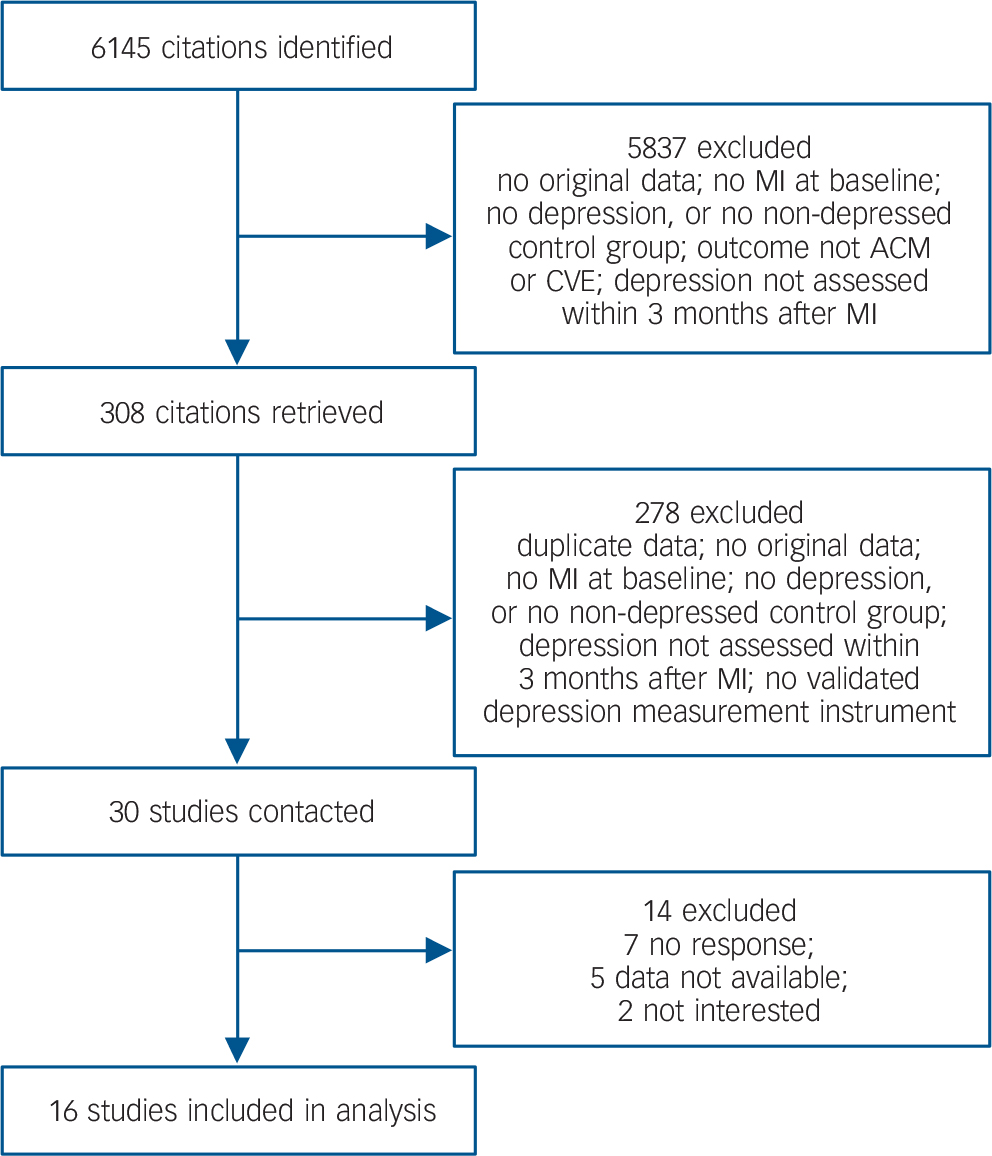
Fig. 1 Study selection and data acquisition. ACM, all-cause mortality; CVE, cardiovascular event; MI, myocardial infarction.
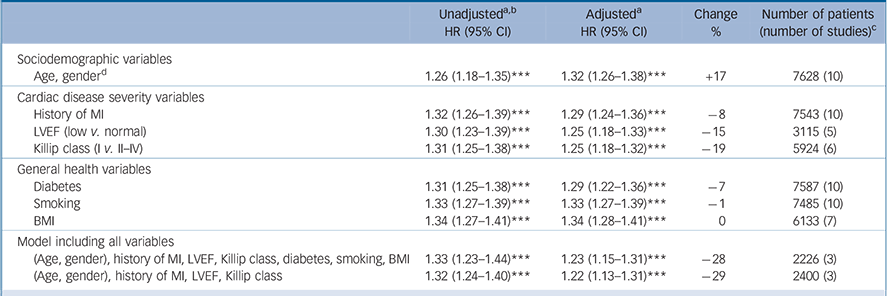
The base Cox regression model for all-cause mortality (adjusted for age and gender) produced a hazard ratio for depression (z-scores) of 1.32 per s.d. (95% CI 1.26-1.38, P<0.001). Adjustment for age and gender increased the strength of the association between post-infarction depression and all-cause mortality by 17% (Table 2). In the cardiovascular events model the hazard ratio (adjusted for age and gender) for depression (z-scores) was 1.19 per s.d. (95% CI 1.14-1.24, P<0.001). Adjustment for age and gender did not substantially alter the strength of the association between post-infarction depression and cardiovascular events (Table 3).
Univariate models. All three cardiac disease severity variables explained a substantial portion of the association between post-infarction depression and all-cause mortality, with the dichotomised variables of Killip class and LVEF explaining 19% and 15% respectively and history of myocardial infarction explaining 8%. Of the general health variables (diabetes, smoking and BMI) only diabetes explained a considerable part (7%) of the association between post-infarction depression and all-cause mortality. Table 2 summarises the unadjusted and adjusted analyses. For cardiovascular events, all three variables relating to cardiac disease severity explained a substantial portion of the association with post-infarction depression, with the dichotomised variables of Killip class and LVEF explaining 12% and 10% respectively and history of myocardial infarction explaining 9%. Of the general health variables again only diabetes explained a considerable part (7%) of the association (Table 3).
Table 1 Patient characteristics at baseline
| Depression group (n = 4043) |
Non-depression group (n = 6132) |
Difference P |
Measured n |
|
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years: mean (s.d.) | 60.5 (12.2) | 61.4 (11.6) | <0.001 | 10 171 |
| Male, % | 63.3 | 75.7 | <0.001 | 10 175 |
| Employed, % | 43.1 | 46.6 | 0.004 | 6528 |
| Living with partner, % | 63.7 | 73.7 | <0.001 | 6412 |
| Cardiac disease severity, % | ||||
| History of MI | 21.8 | 17.0 | <0.001 | 9646 |
| LVEF <40% | 27.0 | 21.3 | 0.001 | 3505 |
| Killip class poor | 21.6 | 15.7 | <0.001 | 7532 |
| PTCA | 48.7 | 43.6 | <0.001 | 7679 |
| History of PTCA | 13.4 | 9.3 | <0.001 | 4830 |
| CABG | 11.5 | 8.5 | <0.001 | 8139 |
| History of CABG | 11.4 | 8.2 | <0.001 | 4849 |
| Thrombolysis | 32.5 | 34.8 | 0.037 | 8065 |
| Congestive HF | 29.0 | 12.3 | <0.001 | 6104 |
| Other risk factors | ||||
| Diabetes, % | 27.8 | 17.3 | <0.001 | 10 060 |
| Smoking, % | 43.1 | 45.6 | 0.017 | 9942 |
| BMI, kg/m2: mean (s.d.) | 27.6 (5.6) | 26.7 (4.5) | <0.001 | 7188 |
| Hyperlipidaemia/hypercholesterolaemia, % | 48.7 | 45.5 | 0.004 | 8405 |
| Hypertension, % | 31.0 | 35.8 | <0.001 | 8301 |
| History of hypertension, % | 55.2 | 44.4 | <0.001 | 5348 |
| Medication use, % | ||||
| Hypolipidaemics | 42.7 | 44.7 | 0.230 | 4004 |
| Beta blockers | 68.7 | 74.0 | <0.001 | 8833 |
| Aspirin | 86.3 | 87.8 | 0.051 | 7561 |
| Calcium channel blockers/antagonists | 19.3 | 14.9 | <0.001 | 7056 |
| ACE inhibitors | 48.1 | 49.6 | 0.160 | 8550 |
| Antidepressants | 10.1 | 3.2 | <0.001 | 5507 |
ACE, angiotensin converting enzyme; BMI, body mass index; CABG, coronary artery bypass graft; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; PTCA, percutaneous transluminal coronary angioplasty.
Multivariable models. The results for all-cause mortality are based solely on the three studies that included all variables (history of myocardial infarction, Killip class, LVEF, diabetes, smoking and BMI). When all the variables were combined in one model, the adjusted HR for all-cause mortality was 1.23 compared with 1.33 unadjusted, an attenuation of 28%. All variables except gender and BMI independently explained part of the association between post-infarction depression and prognosis. Survival curves are shown in Fig. 3(a,b). Adjusting for the three cardiac disease-related variables only, the HR for all-cause mortality was 1.22 compared with 1.32 unadjusted, an attenuation of 29%; this means that these variables are responsible for nearly all of the attenuation in the full model. Model fit improved when age, gender, history of myocardial infarction, LVEF, Killip class and diabetes were subsequently added. Model fit did not improve after further adjustment for BMI and smoking.
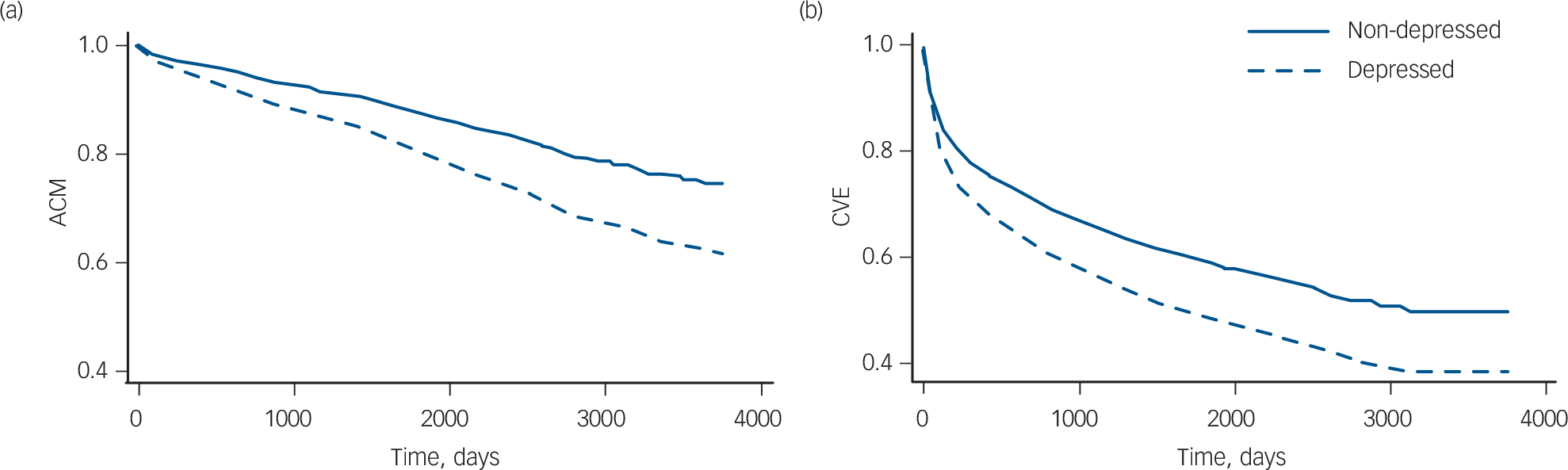
Fig. 2 Survival curves adjusted for age and gender. (a) All-cause mortality (ACM), based on ten studies, n = 7691; (b) cardiovascular events (CVE), based on seven studies, n = 6616.
For cardiovascular events, when combining all general health and disease severity-related variables in one model, the adjusted HR was 1.12 compared with 1.17 unadjusted, an attenuation of 25% (only two studies contained all these variables). All variables except BMI independently explained part of the association between post-infarction depression and prognosis. Survival curves are shown in Fig. 3(c,d). Adjusting for the three cardiac disease-related variables only, the HR for cardiovascular events was 1.13 compared with 1.17 unadjusted, an attenuation of 21%; this means that these variables were responsible for most of the attenuation in the full model. Congruent with the all-cause mortality analyses, model fit improved when subsequently adjusting for age, gender, history of myocardial infarction, LVEF, Killip class and diabetes. Model fit did not improve after further adjustment for BMI and smoking.
Table 2 All-cause mortality: hazard ratios, unadjusted and adjusted for cardiac disease severity and other health-related variables
| UnadjustedFootnote
a
,
Footnote
b
HR (95% CI) |
AdjustedFootnote
a
HR (95% CI) |
Change % |
Number of patients (number of studies)Footnote c |
|
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, genderFootnote d | 1.26 (1.18-1.35)Footnote *** | 1.32 (1.26-1.38)Footnote *** | +17 | 7628 (10) |
| Cardiac disease severity variables | ||||
| History of MI | 1.32 (1.26-1.39)Footnote *** | 1.29 (1.24-1.36)Footnote *** | –8 | 7543 (10) |
| LVEF (low v. normal) | 1.30 (1.23-1.39)Footnote *** | 1.25 (1.18-1.33)Footnote *** | –15 | 3115 (5) |
| Killip class (I v. II-IV) | 1.31 (1.25-1.38)Footnote *** | 1.25 (1.18-1.32)Footnote *** | –19 | 5924 (6) |
| General health variables | ||||
| Diabetes | 1.31 (1.25-1.38)Footnote *** | 1.29 (1.22-1.36)Footnote *** | –7 | 7587 (10) |
| Smoking | 1.33 (1.27-1.39)Footnote *** | 1.33 (1.27-1.39)Footnote *** | –1 | 7485 (10) |
| BMI | 1.34 (1.27-1.41)Footnote *** | 1.34 (1.28-1.41)Footnote *** | 0 | 6133 (7) |
| Model including all variables | ||||
| (Age, gender), history of MI, LVEF, Killip class, diabetes, smoking, BMI | 1.33 (1.23-1.44)Footnote *** | 1.23 (1.15-1.31)Footnote *** | –28 | 2226 (3) |
| (Age, gender), history of MI, LVEF, Killip class | 1.32 (1.24-1.40)Footnote *** | 1.22 (1.13-1.31)Footnote *** | –29 | 2400 (3) |
BMI, body mass index; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
a. Depression is included in all the models as a continuous variable (z-score).
b. Unadjusted HRs are based on analyses including only patients who had scores available for the variables concerned.
c. Depending on availability of these variables in each study.
d. The model including depression, age and gender is the comparison model.
* P<0.05
** P<0.01
*** P<0.001.
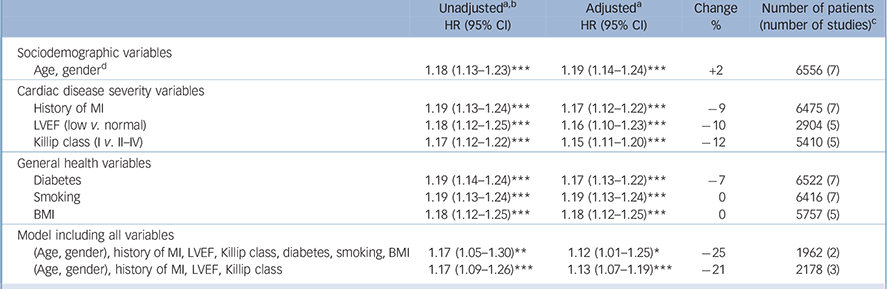
Table 3 Cardiovascular events: hazard ratios, unadjusted and adjusted for cardiac disease severity and other health-related variables
| UnadjustedFootnote
a
,
Footnote
b
HR (95% CI) |
AdjustedFootnote
a
HR (95% CI) |
Change % |
Number of patients (number of studies)Footnote c |
|
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, genderFootnote d | 1.18 (1.13-1.23)Footnote *** | 1.19 (1.14-1.24)Footnote *** | +2 | 6556 (7) |
| Cardiac disease severity variables | ||||
| History of MI | 1.19 (1.13-1.24)Footnote *** | 1.17 (1.12-1.22)Footnote *** | –9 | 6475 (7) |
| LVEF (low v. normal) | 1.18 (1.12-1.25)Footnote *** | 1.16 (1.10-1.23)Footnote *** | –10 | 2904 (5) |
| Killip class (I v. II-IV) | 1.17 (1.12-1.22)Footnote *** | 1.15 (1.11-1.20)Footnote *** | –12 | 5410 (5) |
| General health variables | ||||
| Diabetes | 1.19 (1.14-1.24)Footnote *** | 1.17 (1.13-1.22)Footnote *** | –7 | 6522 (7) |
| Smoking | 1.19 (1.13-1.24)Footnote *** | 1.19 (1.13-1.24)Footnote *** | 0 | 6416 (7) |
| BMI | 1.18 (1.12-1.25)Footnote *** | 1.18 (1.12-1.25)Footnote *** | 0 | 5757 (5) |
| Model including all variables | ||||
| (Age, gender), history of MI, LVEF, Killip class, diabetes, smoking, BMI | 1.17 (1.05-1.30)Footnote ** | 1.12 (1.01-1.25)Footnote * | –25 | 1962 (2) |
| (Age, gender), history of MI, LVEF, Killip class | 1.17 (1.09-1.26)Footnote *** | 1.13 (1.07-1.19)Footnote *** | –21 | 2178 (3) |
BMI, body mass index; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
a. Depression is included in all the models as a continuous variable (z-score).
b. Unadjusted HRs are based on analyses including only patients who had scores available for the variables concerned.
c. Depending on availability of these variables in each study.
d. The model including depression, age and gender is the comparison model.
* P<0.05
** P<0.01
*** P<0.001.
Logistic regression analyses
Five studies (n = 2468) did not have time-to-event data, and therefore logistic regression analyses were performed in addition to the Cox regression analyses. Of the 8366 patients who had depression z-scores as well as outcome data on all-cause mortality, 1136 (14%) died. Of 3206 patients with depression, 636 (12%) died, and of 3206 patients without depression, 500 (16%) died. Of the 8878 patients who had depression z-scores as well as outcome data on cardiovascular events, 3067 experienced a fatal or non-fatal cardiac event (35%). Of 3747 patients with depression, 1449 (39%) experienced such an event, and of 5135 patients without depression, 1168 (32%) experienced an event.
Base model (adjusted for age and gender). The base logistic regression model for all-cause mortality (adjusted for age and gender) produced an OR for depression (z-scores) of 1.41 per s.d. (95% CI 1.34-1.49, P<0.001) (Table 4). Adjustment for age and gender increased the strength of the association between post-infarction depression and all-cause mortality by 18%. The OR for depression in the cardiovascular events model (adjusted for age and gender) for depression (z-scores) was 1.25 per s.d. (95% CI 1.19-1.32, P<0.001) (Table 5). Adjustment for age and gender did not substantially alter the strength of the association between post-infarction depression and cardiovascular events.
Univariate logistic models. For the all-cause mortality and cardiovascular events analyses, the variables diabetes, history of myocardial infarction, LVEF and Killip class each explained a substantial part (5% or more) of the association between post-infarction depression and all-cause mortality and improved model fit. The variables smoking and BMI did not add to the model (Tables 4 and 5).
Multivariable logistic model. When all variables were added to the model at once, 3 studies and 2225 patients remained, and the adjusted OR for depression was 1.24 per s.d. (95% CI 1.07-1.44, P<0.001) in the all-cause mortality model. The added variables explained 30% of the association between post-infarction depression and all-cause mortality. When including the variables relating to cardiac disease severity only (history of myocardial infarction, LVEF and Killip class) the OR was 1.27 per s.d. (95% CI 1.17-1.37, P<0.001). These variables explained 25% of the association (Table 4).
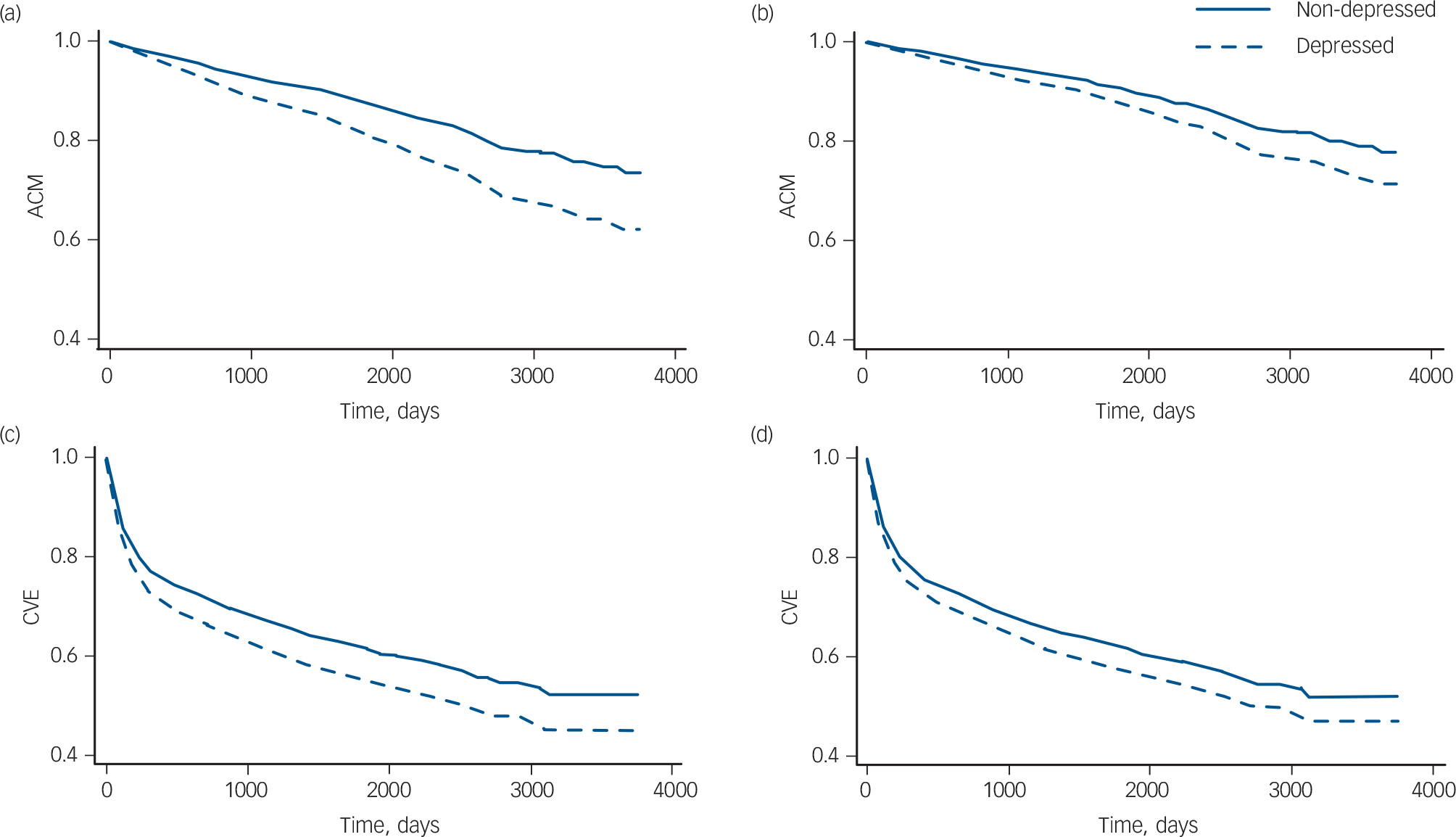
Fig. 3 Survival curves. All-cause mortality (ACM) survival curves based on three studies (n = 2239), (a) adjusted for age and gender, (b) fully adjusted model. Cardiovascular events (CVE) survival curves based on two studies (n = 1973 patients), (c) adjusted for age and gender, (d) fully adjusted model.
The complete model for cardiovascular events included 2 studies and 1964 patients, and resulted in an OR for depression of 1.18 per s.d. (95% CI 1.00-1.39, P = 0.053). The variables explained 23% of the association between post-infarction depression and cardiovascular events. The association was no longer significant after adjusting for all variables. With inclusion of the variables relating to cardiac disease severity only (history of myocardial infarction, LVEF and Killip class) the OR was 1.19 per s.d. (95% CI 1.12-1.26, P<0.001). These variables explained 19% of the association (Table 5).
Participation
Of the 30 studies that were included in the summary data meta-analyses, the authors of 14 studies participated and contributed their data. In addition, 2 studies that were published after the summary data meta-analysis contributed their data. Combining all available studies resulted in inclusion of 51% (10 175 of 19 859) of eligible patients. To estimate the impact of non-participation of studies on the association, 2-year ORs for post-infarction depression were compared for included and excluded studies, as 2-year follow-up data were available for most studies. For comparison purposes this was done on the studies that were included in the original summary data meta-analysis. For excluded studies that reported on all-cause mortality, the unadjusted OR was 1.98 (95% CI 1.62-2.42, P<0.001) and for included studies the unadjusted OR was 2.45 (95% CI 1.46-4.14, P<0.001), with no significant difference. However, for cardiovascular events the ORs of excluded and included studies differed significantly (P = 0.04), with an unadjusted OR of 1.83 (95% CI 1.40-2.39, P<0.001) for excluded studies and 1.34 (95% CI 1.17-1.54, P<0.001) for included studies.
Discussion
The association between post-infarction depression and all-cause mortality and cardiovascular events was partly attenuated - but remained significant - after adjustment for cardiac disease severity and other health variables. In Cox regression analyses, adjusting for cardiac disease severity (history of myocardial infarction, LVEF and Killip class) resulted in an attenuation of 29% in the all-cause mortality model and 21% in the cardiovascular events model. Adjustment for the health-related variables smoking and BMI did not attenuate the association between post-infarction depression and all-cause mortality or cardiovascular events, but adjustment for diabetes attenuated the association for both outcomes by 7%. In logistic regression analyses the results were similar. The fact that after attenuation for variables indicating cardiac disease severity the association between post-infarction depression and prognosis remained can mean several things. First, adjustments for more variables indicating disease severity may result in stronger attenuation. Second, mechanisms other than disease severity are likely to be involved, which may be either mediators in the association between depression and cardiac disease occurring after depression onset, or causal factors preceding both depression and cardiac disease. For example, depression has been associated with changes in autonomic nervous system functioning and in HPA axis activity, Reference Evans, Charney, Lewis, Golden, Gorman and Krishnan11,Reference Carney, Freedland, Rich and Jaffe56,Reference Musselman, Evans and Nemeroff57 increased inflammation, Reference Evans, Charney, Lewis, Golden, Gorman and Krishnan11,Reference Howren, Lamkin and Suls58 and platelet reactivity. Reference Evans, Charney, Lewis, Golden, Gorman and Krishnan11,Reference Musselman, Tomer, Manatunga, Knight, Porter and Kasey59,Reference Laghrissi-Thode, Wagner, Pollock, Johnson and Finkel60 These physiological processes may be particularly disturbed in patients with depression following myocardial infarction, and they are all involved in the development and progression of cardiovascular disease. They may therefore be mediating mechanisms through which depression in these patients can affect prognosis. These processes may, however, also be involved in both the onset of depression and of cardiac disease progression, in which case they are confounders of the association between post-infarction depression and prognosis.
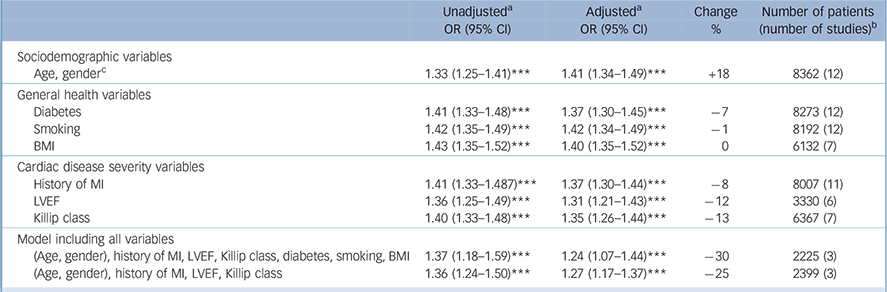
Table 4 All-cause mortality: odds ratios for depression, unadjusted and adjusted for cardiac disease severity and health-related variables
| UnadjustedFootnote
a
OR (95% CI) |
AdjustedFootnote
a
OR (95% CI) |
Change % |
Number of patients (number of studies)Footnote b |
|
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, genderFootnote c | 1.33 (1.25-1.41)Footnote *** | 1.41 (1.34-1.49)Footnote *** | +18 | 8362 (12) |
| General health variables | ||||
| Diabetes | 1.41 (1.33-1.48)Footnote *** | 1.37 (1.30-1.45)Footnote *** | –7 | 8273 (12) |
| Smoking | 1.42 (1.35-1.49)Footnote *** | 1.42 (1.34-1.49)Footnote *** | –1 | 8192 (12) |
| BMI | 1.43 (1.35-1.52)Footnote *** | 1.40 (1.35-1.52)Footnote *** | 0 | 6132 (7) |
| Cardiac disease severity variables | ||||
| History of MI | 1.41 (1.33-1.487)Footnote *** | 1.37 (1.30-1.44)Footnote *** | –8 | 8007 (11) |
| LVEF | 1.36 (1.25-1.49)Footnote *** | 1.31 (1.21-1.43)Footnote *** | –12 | 3330 (6) |
| Killip class | 1.40 (1.33-1.48)Footnote *** | 1.35 (1.26-1.44)Footnote *** | –13 | 6367 (7) |
| Model including all variables | ||||
| (Age, gender), history of MI, LVEF, Killip class, diabetes, smoking, BMI | 1.37 (1.18-1.59)Footnote *** | 1.24 (1.07-1.44)Footnote *** | –30 | 2225 (3) |
| (Age, gender), history of MI, LVEF, Killip class | 1.36 (1.24-1.50)Footnote *** | 1.27 (1.17-1.37)Footnote *** | –25 | 2399 (3) |
BMI, body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
a. Depression is included in all the models as a continuous variable (z-score).
b. Depending on availability of these variables in each study.
c. The model including depression, age and gender is the comparison model.
* P<0.05
** P<0.01
*** P<0.001.
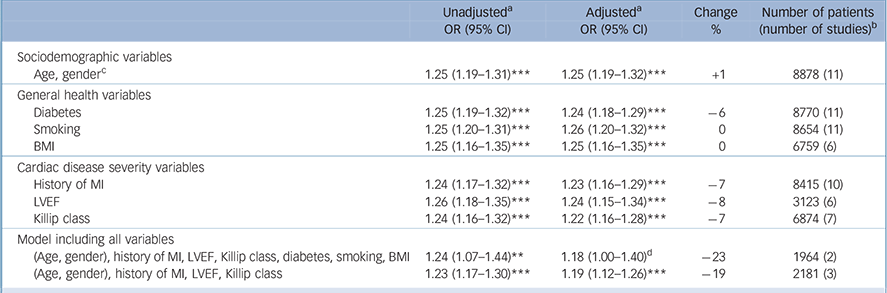
Table 5 Cardiovascular events: odds ratios for depression, unadjusted and adjusted for cardiac disease severity and health-related variables
| UnadjustedFootnote
a
OR (95% CI) |
AdjustedFootnote
a
OR (95% CI) |
Change % |
Number of patients (number of studies)Footnote b |
|
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, genderFootnote c | 1.25 (1.19-1.31)Footnote *** | 1.25 (1.19-1.32)Footnote *** | +1 | 8878 (11) |
| General health variables | ||||
| Diabetes | 1.25 (1.19-1.32)Footnote *** | 1.24 (1.18-1.29)Footnote *** | –6 | 8770 (11) |
| Smoking | 1.25 (1.20-1.31)Footnote *** | 1.26 (1.20-1.32)Footnote *** | 0 | 8654 (11) |
| BMI | 1.25 (1.16-1.35)Footnote *** | 1.25 (1.16-1.35)Footnote *** | 0 | 6759 (6) |
| Cardiac disease severity variables | ||||
| History of MI | 1.24 (1.17-1.32)Footnote *** | 1.23 (1.16-1.29)Footnote *** | –7 | 8415 (10) |
| LVEF | 1.26 (1.18-1.35)Footnote *** | 1.24 (1.15-1.34)Footnote *** | –8 | 3123 (6) |
| Killip class | 1.24 (1.16-1.32)Footnote *** | 1.22 (1.16-1.28)Footnote *** | –7 | 6874 (7) |
| Model including all variables | ||||
| (Age, gender), history of MI, LVEF, Killip class, diabetes, smoking, BMI | 1.24 (1.07-1.44)Footnote ** | 1.18 (1.00-1.40)Footnote d | –23 | 1964 (2) |
| (Age, gender), history of MI, LVEF, Killip class | 1.23 (1.17-1.30)Footnote *** | 1.19 (1.12-1.26)Footnote *** | –19 | 2181 (3) |
BMI, body mass index; LVEF, left ventricular ejection fraction; MI, myocardial infarction.
a. Depression is included in all the models as a continuous variable (z-score).
b. Depending on availability of these variables in each study.
c. The model including depression, age and gender is the comparison model.
d. P = 0.053.
* P<0.05
** P<0.01
*** P<0.001.
In addition to physiological mechanisms, behavioural mechanisms may be involved as mediators. Depression following myocardial infarction has been associated with a range of behaviours that are unhealthy and may increase the risk of mortality and new cardiac events. Patients with depression have poor medication adherence, Reference Rieckmann, Kronish, Haas, Gerin, Chaplin and Burg61-Reference May, Sheng, Catinella, Horne, Carlquist and Joy63 and low adherence to rehabilitation programmes. Reference Blumenthal, Williams, Wallace, Williams and Needles64 Moreover, they display more generally unhealthy behaviours, such as maintaining a high-fat diet, smoking and a lack of physical exercise. Reference Myers, Gerber, Benyamini, Goldbourt and Drory65-Reference Ziegelstein, Fauerbach, Stevens, Romanelli, Richter and Bush67 These unhealthy behaviours may be the result of psychological mechanisms associated with depression. For example, low self-efficacy in such patients may imply they are less likely to believe they can control and influence their prognosis, for example by changing unhealthy behaviour patterns. In a sample of patients with heart failure, low self-efficacy was associated with poor adherence, Reference Schweitzer, Head and Dwyer68 and in another study low self-efficacy predicted poor self-management behaviours in patients with myocardial infarction. Reference Joekes, Van Elderen and Schreurs69 Interestingly, self-efficacy appears to be associated with poor adherence and health status independently of depression. Reference Schweitzer, Head and Dwyer68-Reference Sarkar, Ali and Whooley70 However, evidence also suggests that the association between self-efficacy and worsened prognosis is caused by worse cardiac disease severity in patients with low self-efficacy, Reference Sarkar, Ali and Whooley71 which is consistent with the confounding role of disease severity in the association between depression and prognosis.
The attenuation after adjusting for LVEF and Killip class was somewhat stronger for all-cause mortality than for cardiovascular events in both time-to-event (LVEF 15% v. 10%, Killip class 19% v. 12%) and logistic (LVEF 12% v. 8%, Killip class 13% v. 7%) regression analyses. This is unexpected, as these variables appear to be more strongly related to cardiac disease than to all-cause mortality and would therefore be expected to explain more of the association between depression and cardiac outcomes than of the association between depression and all-cause mortality. Potentially, however, poor Killip class and low LVEF - symptoms of heart failure - are markers of poor health in general. Heart failure is often comorbid with other serious health problems such as lung disease, obesity and diabetes, with 40% of people with heart failure having more than five comorbid conditions. Reference Page and Lindenfeld72 Any of these health problems might escalate and cause mortality, not just cardiac disease. Reference Page and Lindenfeld72 This might explain why adjusting for LVEF and Killip class attenuates the association between depression and all-cause mortality more than it does the association between depression and cardiovascular events, as patients with low LVEF or Killip class have a higher risk of dying early of any cause, not just cardiac causes, than do patients with normal LVEF or Killip class.
Results in the context of previous studies
The attenuation of the association between post-infarction depression and cardiac prognosis after adjustment is a consistent finding in studies in this field, Reference Nicholson, Kuper and Hemingway4,Reference Carney, Blumenthal, Catellier, Freedland, Berkman and Watkins15-Reference Parashar, Rumsfeld, Spertus, Reid, Wenger and Krumholz17 but identifying the factors that cause this attenuation has proved to be difficult thus far. In the summary data meta-analysis preceding this IPD meta-analysis, Reference Meijer, Conradi, Bos, Thombs, van Melle and de Jonge5 reported analyses adjusting for a number of factors including variables relating to disease severity attenuated the association between post-infarction depression and prognosis by on average 21%. However, adjusted analyses were reported in a limited number of studies only, using different sets of variables, and adjustment was made for several variables at once, making it impossible to see to what extent individual variables were responsible for attenuation. The fully adjusted model in this IPD meta-analysis resulted in slightly higher attenuation than the summary data meta-analysis. Similar, but greater, attenuation was found in another summary data meta-analysis by Nicholson et al. Reference Nicholson, Kuper and Hemingway4 They reported that a pooled OR of adverse outcomes in depressed v. non-depressed myocardial infarction patient groups, adjusted for diverse variables, was attenuated by 41%. So overall, it appears that in this study we have identified the disease-specific variables that are responsible for the largest portion of the attenuation known so far.
The summary data meta-analysis preceding this IPD meta-analysis resulted in ORs of 2.25 for all-cause mortality, 2.71 for cardiac mortality and 1.59 for cardiovascular events. Similar effect sizes were reported in other meta-analyses. Reference Barth, Schumacher and Herrmann-Lingen3,Reference Nicholson, Kuper and Hemingway4 Our meta-analysis resulted in HRs of 1.32 for all-cause mortality and 1.19 for cardiovascular events. This apparent difference in the effect size is mainly due to the fact that these ratios were based on different analyses. The associations in the previous meta-analysis were based on (maximally) 2-year follow-up data, dichotomised depression scores and logistic regression analysis. The main associations in our meta-analysis are based on longer follow-up data, continuous depression scores and Cox regression analyses. In previous meta-analyses dichotomous depression scores were used, so the effect sizes represented the increase in risk associated with the difference between patient groups with and without depression, which is a large difference. With the use of continuous depression scores, the hazard ratios in this IPD meta-analysis represent the increase in risk associated with 1 s.d. increase in continuous depression scores, so the steps involved are smaller and results are more precise. All these differences, and the fact that the risk of spurious results due to low numbers of events is small in the IPD meta-analysis, explain these apparently different results in the IPD meta-analysis compared with the summary data meta-analysis. As depression is by nature a continuous variable instead of a dichotomous variable, expressing the effect on prognosis per s.d. is more accurate than expressing it dichotomously.
Strengths and limitations
Individual studies often lack power to adjust for several variables, and summary data meta-analyses are limited to combining reported data. Instead, combining individual patient data provides more statistical power, consistent analysis of data across studies and the possibility of additional analyses not performed in the original studies. Most important for this study is that IPD meta-analysis allowed us to investigate in detail some of the variables responsible for the attenuation of the association between post-infarction depression and prognosis. In addition, time-to-event analyses could be performed. As time-to-event analyses combine the occurrence and timing of events, Reference Van Walraven25 they are more reliable and stable than (for example) odds ratios, which are often used in summary data meta-analyses. This resulted in a relatively accurate estimate of the effect of cardiac disease severity on the association between post-infarction depression and prognosis.
There were also a number of limitations to this study. First, the analyses were to some extent limited in power, as not all studies participated, and not all of those that did reported the variables we selected for analysis. For example, for analyses with Killip class and LVEF, only six and five studies respectively were available. However, these analyses were still based on a large number of patients, and there is no other way to perform such analyses. Therefore they do have an added value in summarising the role of these variables in the association between post-infarction depression and prognosis.
With regard to participation, of 30 eligible studies, 16 were included, of which the majority were from Western high-income countries. Although low, this level of participation is common for IPD meta-analyses of observational studies; Reference Cooper and Patall21 Schmid et al achieved participation by 11 of the 14 researchers of clinical trials, Reference Schmid, Landa, Jafar, Giatras, Karim and Reddy73 but Sternberg et al achieved participation by 13 of 24 (54%) observational studies, Reference Sternberg, Baradaran, Abbott, Lamb and Guterman74 and Wicherts et al only 64 of 249 studies (26%). Reference Wicherts, Borsboom, Kats and Molenaar75 For cardiovascular events the odds ratios in eligible non-included studies were higher than those in included studies (1.83 v. 1.34). This suggests that, for this outcome, the IPD meta-analysis potentially underestimates the strength of the association. However, the higher, more extreme odds ratios in the non-included studies came from the smaller studies. These, if included, would have had a relatively smaller effect on the overall combined OR than the larger included and non-included studies, in which the ORs were more moderate. In addition, there was no other appreciable difference between the eligible studies that were included and those that were excluded. We therefore concluded that the data available for this IPD meta-analysis, and the results of the analysis, are fairly representative of people experiencing myocardial infarction in high-income countries, and that the benefits of analysing IPD outweighed the potential effects of excluding relevant studies.
Two of the included studies (ENRICHD and MIND-IT; see online Table DS1) were trials of depression treatment. As these studies collected information on both depression and outcomes, they were considered relevant for answering our research question. Their data may differ from those of observational studies, as patients were treated for depression, which may affect depression as well as all-cause mortality and cardiovascular outcomes. However, the depression scores that were included in the analyses for this study were obtained before treatment started and were therefore not affected by the interventions. In addition, the interventions were at best moderately effective in reducing depression and did not have an effect on all-cause mortality and cardiovascular outcomes. Reference Berkman, Blumenthal, Burg, Carney, Catellier and Cowan76-Reference Van Melle, de Jonge, Honig, Schene, Kuyper and Crijns78
An inherent problem of IPD meta-analysis is that individual studies use different methods to assess relevant variables. In our meta-analysis variables were harmonised across studies, with the main issue being depression measurement. However, such harmonisation always contains the risk of combining measurements based on different underlying constructs. Reference Bauer and Hussong79 For depression, however, most studies used the BDI, making their data comparable. The other questionnaires used were the BDI-FS, HADS-D and Zung SDS, which are highly correlated with the BDI. Item response theory methods of harmonisation could not be used here, as they require individual depression item scores, which were not available for 4 of the 16 studies. Standardising depression scores within each study might introduce bias by levelling out any differences in depression severity and prevalence between studies, which might affect the strength of the association between post-infarction depression and prognosis. If the strength of the association were different for patients with less severe v. more severe depression, this would not be accounted for by these standardisation methods. We therefore investigated whether this bias was present in our study by adding a variable to the model that describes the level of depression per study (percentage depressed based on depression questionnaire scores), as well as an interaction variable of the standardised depression scores and the percentage depressed variable. If this interaction variable were a significant predictor in the model, this would mean that the association between depression and prognosis was moderated by level of depression. This, however, was not the case (HR = 1.00, 95% CI 0.99-1.01, P = 0.9). We are therefore confident that this potential bias did not affect our results.
Although LVEF, history of infarction and Killip class are important predictors of post-infarction mortality and morbidity, they are not the only risk factors associated with worsened prognosis. Other important variables that are related to disease severity, for example heart rate, blood pressure and treatment with percutaneous transluminal coronary angioplasty or CABG, Reference Eagle, Lim, Dabbous, Pieper, Goldberg and Van de Werf80 could not be included in the analyses. Similarly, a number of non-cardiac comorbidities, such as chronic obstructive pulmonary disease and kidney disease, are known to be associated with worsened outcomes. Reference Wakabayashi, Gonzalez, Delhaye, Ben-Dor, Maluenda and Collins81 Also, the type of treatment in the acute phase and subsequent pharmacological treatment can affect outcomes. Finally, behavioural variables that were not incorporated in the model can attenuate the association. In a sample of patients with stable coronary artery disease, Whooley et al found that physical activity was an important confounder of the association. Reference Whooley, de Jonge, Vittinghoff, Otte, Moos and Carney37 Adding these variables to a prediction model is likely to result in additional attenuation of the association between post-infarction depression and prognosis. However, related research suggests the association may remain: Kronish et al, for example, found that depression after acute coronary syndrome remained associated with all-cause mortality, even after adjusting for Global Registry of Acute Coronary Events (GRACE) risk score and LVEF, Reference Kronish, Rieckmann, Schwartz, Schwartz and Davidson82 and Meurs et al found similar results after adjusting the association for GRACE risk score in patients with myocardial infarction. Reference Meurs, Zuidersma, Dickens and de Jonge83
Finally, the outcome of all-cause mortality is somewhat imprecise in investigations of the association between post-infarction depression and prognosis. First, in some studies cardiac deaths may be included in the outcome ‘cardiovascular events’ as well as in the outcome all-cause mortality. Therefore, the results of the analyses for the two outcome measures are based in part on the same event data. Second, as all-cause mortality includes deaths from non-cardiac causes, the results of this study might encompass a part of the association between post-infarction depression and prognosis that is not specific to cardiac disease. However, the reason to use all-cause mortality was that the outcome data available in studies of post-infarction depression often include mortality without specified causes of death. This was preferred over excluding studies without cardiac-specific outcome measures, which would have considerably reduced the number of available studies and otherwise relevant data. In addition, all-cause mortality includes cardiac mortality, and data on cardiac mortality and morbidity were analysed separately to obtain results that were maximally specific. As all-cause mortality includes non-cardiac mortality, the proportion of variance in the association between post-infarction depression and all-cause mortality explained by cardiac disease severity might be smaller than it would be if only cardiac mortality were included. This is because part of the association between post-infarction depression and all-cause mortality is non-specific for cardiac disease and may therefore not be affected by adjusting for cardiac disease-related variables.
Implications of the study
This study represents an important step forward in understanding the association between post-infarction depression and prognosis, as it is the first time that the amount of attenuation of this association by cardiac disease severity has been systematically quantified. Therefore, an important part of the inconsistencies in previous literature, due to conflicting results and methodological issues, has been solved. It appears more severe cardiac disease is a common underlying factor resulting in both poorer prognosis and higher risk of depression. In addition, however, post-infarction depression remains independently associated with poorer cardiac prognosis, despite this attenuation. This means either that it is depression itself that adversely affects outcomes, or that unknown mechanisms can further explain the association.
Future research should focus not only on the mechanisms through which post-infarction depression is associated with poorer cardiac outcomes, but also on better ways to treat such depression. As depression is widely recognised to be an extremely heterogeneous concept, with many different causes, symptom profiles and clinical courses, there currently is a movement towards more individualised treatments. Within such individualised depression care for patients following myocardial infarction, integrating depression treatment and treatment of major indicators of cardiac disease severity could help improve prognosis, which post-infarction depression treatments as yet have not achieved.
Acknowledgements
We acknowledge Dr B. D. Thombs and Dr R. C. Ziegelstein for their critical review of the paper, and R. B. K. Wanders for input in statistical matters. Additional researchers involved in individual studies were Johan Ormel, PhD, Interdisciplinary Centre for Psychiatric Epidemiology, University Medical Centre Groningen, Groningen, The Netherlands; Serena Bergerone, MD, Division of Cardiology, Department of Internal Medicine, San Giovanni Battista Hospital, University of Turin, Turin, Italy; Lisa Berkman, PhD, Centre for Population and Development Studies, Harvard University, Boston, USA; Hannah McGee, BA, PhD, Division of Population Health Sciences (Psychology), Ronán Conroy, BA, Division of Population Health Sciences (Epidemiology and Public Health Medicine), Royal College of Surgeons in Ireland, Dublin, Ireland; and Roy Ziegelstein, MD, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, USA











eLetters
No eLetters have been published for this article.