Introduction
Successful conservation work of migratory birds needs to consider the complete annual cycle and spatial distribution of the population (Schuster et al. Reference Schuster, Wilson, Rodewald, Arcese, Fink, Auer and Bennett2019). Use of individual tracking with, for example, Global Positioning System (GPS) tags, has revolutionised our ability to remotely detect migration routes (Bridge et al. Reference Bridge, Thorup, Bowlin, Chilson, Diehl, Fléron, Hartl, Roland, Kelly, Robinson and Wikelski2011), key resting sites, and details about how birds utilise sites at fine spatial scale (Si et al. Reference Si, Xu, Xu, Zhang, Wielstra, Wei, Liu, Luo, Takekawa, Balachandran, Zhang, de Boer, Prins and Gong2018). Even in small populations, in the spotlight of dedicated conservationists, studies based on reports of flocks or individually marked birds have the limitation that recoveries are biased towards already known sites (Fancy Reference Fancy, Pank, Douglas, Curby and Garner1988). Remote tracking allows detection even at sites where observers are missing. This is not only an advantage in remote areas (Zhang et al. Reference Zhang, Xie, Li, Batbayar, Deng, Damba and Fox2020), but also where small groups of individuals of special interest are hidden in large flocks of similar species, making them hard to find.
The Lesser White-fronted Goose (Anser erythropus) is the most threatened goose species of the northern hemisphere and the western Palearctic (Heredia et al. Reference Heredia, Rose and Painter1996; Jones et al. Reference Jones, Martin, Barov and Nagy2008). Its historic breeding distribution included Scandinavia and continued all the way to Chukotka in eastern Russia. However, during their population decline, most pronounced in 1930–1960, the species’ distribution was fragmented into three different populations (Jones et al. Reference Jones, Martin, Barov and Nagy2008). These were mainly delimited by their breeding sites and migration routes: (i) the Fennoscandian population breeding in northern Sweden, Norway, and Finland; (ii) the western main population breeding from Yamal to the Taimyr Peninsula; (iii) the eastern main population breeding in the Russian tundra east of Taimyr (Jones et al. Reference Jones, Martin, Barov and Nagy2008, Morozov Reference Morozov1995, Madsen et al. Reference Madsen, Cracknell and Fox1999). During the twentieth century, the Fennoscandian population suffered dramatic declines to the extent that in the 1980s only 60–90 breeding pairs remained, including 20 pairs breeding in the Swedish mountain tundra (Norderhaug and Norderhaug Reference Norderhaug and Norderhaug1984). This decline was mainly explained by increased mortality, most likely due to increased disturbance levels in the breeding grounds, habitat degradation in wintering sites in south-east Europe (mainly Hungary and Greece), and overharvesting during migration (e.g. Madsen et al. Reference Madsen, Cracknell and Fox1999). Today, breeding Lesser White-fronted Geese in Fennoscandia are known from only two distinct areas on high-altitude mountain tundra, one in northern Norway and another in Swedish Lapland (Staneva and Burfield Reference Staneva and Burfield2017).
As a response to the dwindling population numbers, plans for an ex situ breeding programme were initiated in Sweden in the late 1970s (von Essen, Reference von Essen1991). From 1981 to 1999, a total of 341 birds were released to reinforce the remnant breeding population in Swedish Lapland (Andersson and Holmqvist Reference Andersson and Holmquist2010). The young Lesser White-fronted Geese were released with adoptive Barnacle Geese (Branta leucopsis) parents as a measure to change the population’s migration traditions on to safer routes and wintering sites. Barnacle Geese used by the programme originated from a semi-domesticated population that was known to winter in the Netherlands (von Essen Reference von Essen1991). According to knowledge about imprinting of geese in the 1970s, the released birds were expected to follow their foster parents to wintering grounds and the next year their inherent strong site fidelity would guide them back to the release sites (Andersson Reference Andersson2019). This objective of this unorthodox conservation measure was to avoid the high mortality found along the eastern migration routes (von Essen Reference von Essen1991, Reference von Essen1997, Reference von Essen1999). Reports of colour-ringed released Lesser White-fronted Geese indicated that the programme was successful in that the majority of released birds in Sweden were found wintering in the Netherlands in subsequent years (Mooij et al. Reference Mooij, Hansson, Kampe-Persson and Nilsson2008).
So far, knowledge about the movements of the reinforced Lesser White-fronted Goose population breeding in Sweden has mainly been based on reports of ringed birds and includes evident temporal gaps (SEPA 2011). We know, for example, that from the breeding grounds the birds first migrate south along the Baltic coast and use resting sites near Hudiksvall and Uppsala. Later in September they were regularly observed in two larger areas in the Netherlands, where the geese also spent the winter (Koffijberg et al. Reference Koffijberg, Cottaar and van der Jeugd2005). Furthermore, the migration of the reinforced population and possible impact on migration traditions of other Lesser White-fronted Geese caused debate even though movements of Swedish birds have not been known in detail (Marchant and Musgrove Reference Marchant and Musgrove2011, Reinert Reference Reinert2019). Therefore, we initiated the use of GPS tracking, previously used for the conservation of other Lesser White-fronted Goose populations (Aarvak and Øien Reference Aarvak and Øien2003, Lei et al. Reference Lei, Jia, Zuo, Zeng, Shi, Zhou and Wen2019, Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen, Lei, Lu, Bridgewater, Lei and Zeng2021). By detailed tracking of Swedish Lesser White-fronted Geese we aimed to identify the full network of stopover sites used by the population to provide an overview of site-protection status and implications for conservation within the EU.
Methods
In 2015 and 2016, we caught four Lesser White-fronted Geese on a spring stopover site in Hudiksvall, Sweden (61°44’ N / 27°06’ E). Three of the birds were tagged in May 2015, two of which (males Niklas and John) were tagged with GPS–Global System for Mobile communication (GSM) transmitters (25 g) (Microwave Ltd), while one bird (female Nina; paired with Niklas) was tagged with a high-resolution data logger with ultra-high frequency (UHF) download (25 g) (e-obs GmbH, Grünwald, Germany) (see Table 1). In May 2016, we recaptured Nina and removed her tag. On the same day, another female (Hanna) was caught and tagged with a GPS–General Packet Radio Services (GPRS) transmitter (22 g) (madebytheo). All tags were equipped with solar panels and fixed on the birds as backpacks with a special Teflon harness (Lameris et al. Reference Lameris, Kölzsch, Dokter, Nolet and Müskens2017). Each tagged bird was observed during autumn migration to verify breeding success.
Table 1. Technical parameters of tags and analysed data sets.
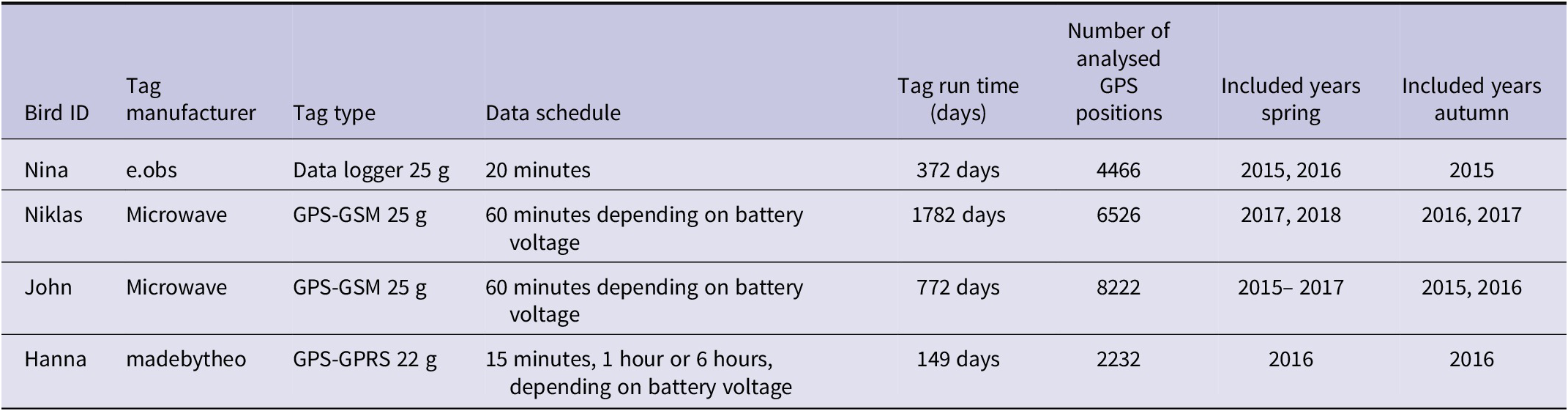
All data were uploaded to Movebank (www.movebank.org) (Kays et al. Reference Kays, Davidson, Berger, Bohrer, Fiedler, Flack, Hort, Hahn, Gauggel, Russell, Kölzsch, Lohr, Partecke, Quetting and Safi2022); the tracks were of lengths between 149 days (Hanna) and almost 5 years (Niklas) (see Table 1). As Niklas and Nina formed a pair, we used only the data from Nina during 2015/6 to keep data sets independent when both partners were carrying a transmitter (Table 1). For each year, we divided the data into spring migration (1 March–1 June) and autumn migration (1 July–1 October). Note that autumn migration thus included pre- and post-moult movements. All locations with error estimates >100 m were excluded and the resolution was thinned to one location per hour. The data selection and cleaning was carried out using the Apps “Filter by Season” and “Thin by Time”, included in the MoveApps workflow “Migration flyway outlines with dynamic Brownian Bridge Movement Model (dBBMM)” (Kölzsch et al. Reference Kölzsch, Moonen and Scharf2022). By estimating the length of staging within the breeding area (June–August), we determined breeding status (26 days incubation, 5–6 weeks brood rearing). Starting moult migration was a clear indication for none or failed breeding.
For the two migration seasons, we used dynamic dBBMMs to identify high-density use stopover sites; see “dyn Brownian Bridge” App in MoveApps workflow (Kölzsch et al. Reference Kölzsch, Moonen and Scharf2022). dBBMM is a random movement model that estimates time, distance, and behaviour-dependent random movement between successive pairs of locations (Horne et al. Reference Horne, Garton, Krone and Lewis2007, Walter et al. Reference Walter, Fischer, Baruch-Mordo and Vercauteren2011). Within the statistical program R (R Core Team 2021), we used the package “move” (Kranstauber et al. Reference Kranstauber, Kays, LaPoint, Wikelski and Safi2012) to create individual dBBMMs for spring and autumn migration (function “brownian.bridge.dyn”, window.size: 31, margin: 11). The resulting individual Brownian bridge utility distributions were then combined to one such distribution for all individuals combined for both spring and autumn migration. Finally, we calculated contour lines of the combined utility distribution volumes that indicate minimum areas in which a bird is present during the considered time period with a certain, user-defined probability. We selected the probabilities 0.5, 0.95, 0.99, and 0.999 (i.e. 50%, 95%, 99%, and 99.9%) for our analyses. For example, the contour line of 99.9% shows the area where a Lesser White-fronted Goose of our population could be found during the respective migration interval with extremely high certainty.
We manually examined all sites indicated by the 99% and 95% contour lines and extracted entry and exit timestamps in small-scale areas of radius <1 km, where birds did not fly (GPS speed <1 m/s) and stayed on the ground. Entry and exit locations were considered with a temporal uncertainty due to the fixing schedule of the tags. Staging durations were calculated as difference between the first and last location in the site for each individual. If several individuals rested in an area or if the area was used for several years by one individual, mean durations with standard deviations (SDs) were calculated.
All sites (including wintering, 1 October–1 March) were characterised as being within or outside a Special Protected Area (SPA), including the Natura 2000 network, and if the species was listed (yes/no) as of special concern (https://natura2000.eea.europa.eu). By scanning project databases (www.geese.org, internal database Projekt Fjällgås, see, e.g. Liljebäck et al. Reference Liljebäck, Koffijberg, Kowallik, Månsson and Andersson2021) for earlier records of colour-ringed birds and revisiting listed relevant sources below we determined if the identified site was known to host Lesser White-fronted Geese.
Sweden: Andersson et al. (Reference Andersson2019); https://artfakta.se/artbestamning/taxon/anser-erythropus-100008, reports in national reporting gateway www.artportalen.se
Germany: Kruckenberg and Krüger (Reference Kruckenberg and Krüger2013), Mooij and Heinicke (Reference Mooij and Heinicke2008), reports in national reporting gateway www.ornitho.de
The Netherlands: Koffijberg. and van Winden (Reference Koffijberg and van Winden2013), Koffijberg et al. (Reference Koffijberg, Cottaar and van der Jeugd2005), reports in national reporting gateway https://waarneming.nl/
Results
All four tracked Lesser White-fronted Geese visited the core breeding area in Swedish Lapland and migrated to winter in the Netherlands. According to the data, the migration corridor led along the Swedish east coast, crossing the Baltic Sea, following the German and Dutch North Sea coast, and arriving at the wintering sites in the south-west of the Netherlands. However, diverging parts of the migration trajectory were also identified, which covered unexpected areas in Denmark and Finland during autumn and Norway in spring (Figure 1).
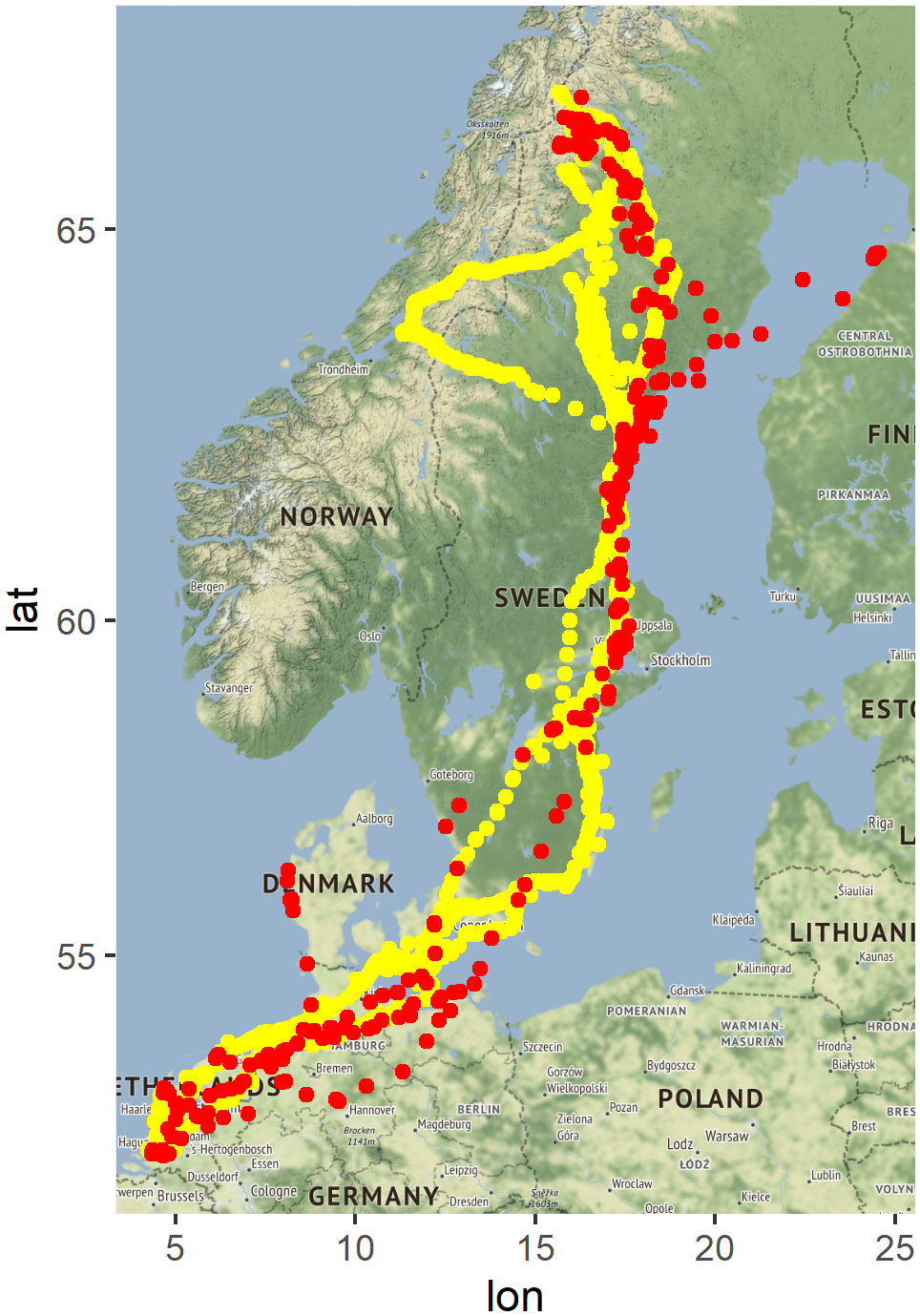
Figure 1. GPS fixes of the four tagged wild Lesser White-fronted Geese caught in Sweden (2014–2019). Colours indicate if the position was retrieved during autumn migration (red) or spring migration (yellow).
The individual and combined dBBMM results (see Figure 2 for the combined dBBMMs) provided information about the areas that were consistently used by our tracked birds and the Swedish breeding population of Lesser White-fronted Geese in general. The area inside individual contours had a low SD and did not differ from the combined contours of both migrations (spring: individual mean ± SD: 2,868,800 ± 530 km2, combined: 2,867,791 km2, Wilcoxon signed-rank test P = 0.22; autumn: individual mean ± SD: 2,811,073 ± 858 km2, combined: 2,810,170 km2, Wilcoxon signed-rank test P = 0.50), indicating that the migration corridors were relatively similar between individuals and years. However, the combined area was consistently larger during spring (Wilcoxon signed-rank test P <0.001), which agrees with the longer migration duration and more numerous spring stopover sites. In detail, spring migration (Figure 2a) was initiated by departure from the wintering grounds in the Netherlands, followed by non-stop flight either along the North Sea coast or through inland the Netherlands/Germany to the Danish island of Lolland (site 9, Figure 2c, Table 2). This site was not known before and is of high importance for the population as birds used it for about four weeks. From this stepping stone, two alternative routes led the birds north-east, one following the Baltic coast, including several short stopovers, the other leading non-stop over inland Sweden. Flocks following the different routes interlaced south of Stockholm, primarily at Svartåmynningen (site 8). All birds in all years later visited sites along the coast of the Bothnian Sea (sites 5 and 6). The last phase of spring migration showed a high degree of variation in route choice (three clear, up to 230 km distant paths in spring vs only 1–2 closer paths during other parts of migration), but all routes re-joined at pre-breeding sites close to Ammarnäs, Båtsjaur, and Kaskeloukt (sites 2, 3, and 4). Notably, one spring migration track led directly to the breeding area (site 1), without any pre-breeding stops, and this led to the only successful breeding attempt made by one of our tracked birds (see Table 1, bird John in 2016). Breeding success has been verified by the length of stay in the breeding area (incubation 26 days plus brood-rearing), as well as visual observations of the families on autumn migration.
Table 2. Sites of Lesser White-fronted Geese identified by our analysis (see Figures 2a and b); ID, name, and country (SE–Sweden, DK–Denmark, DE–Germany, NL–Netherlands), season and site use, staging duration (mean ± SD), site protection status according to SPA legislation (see Figure 2c), and an indication if protection measures in the SPAs are also directed towards Lesser White-fronted Geese. Bold indicates sites where the presence of Lesser White-fronted Geese was previously unknown. SPA, Special Protected Area.
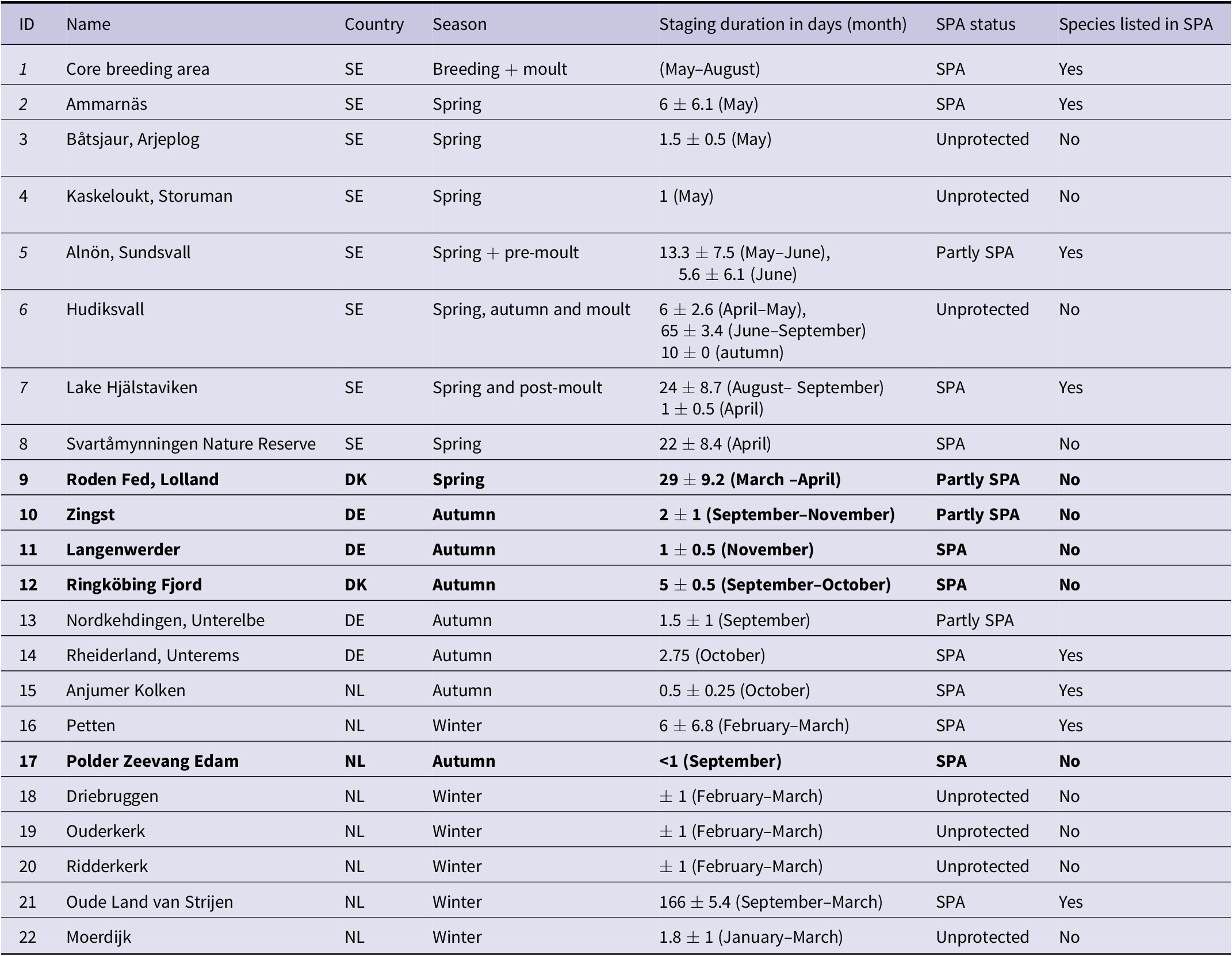
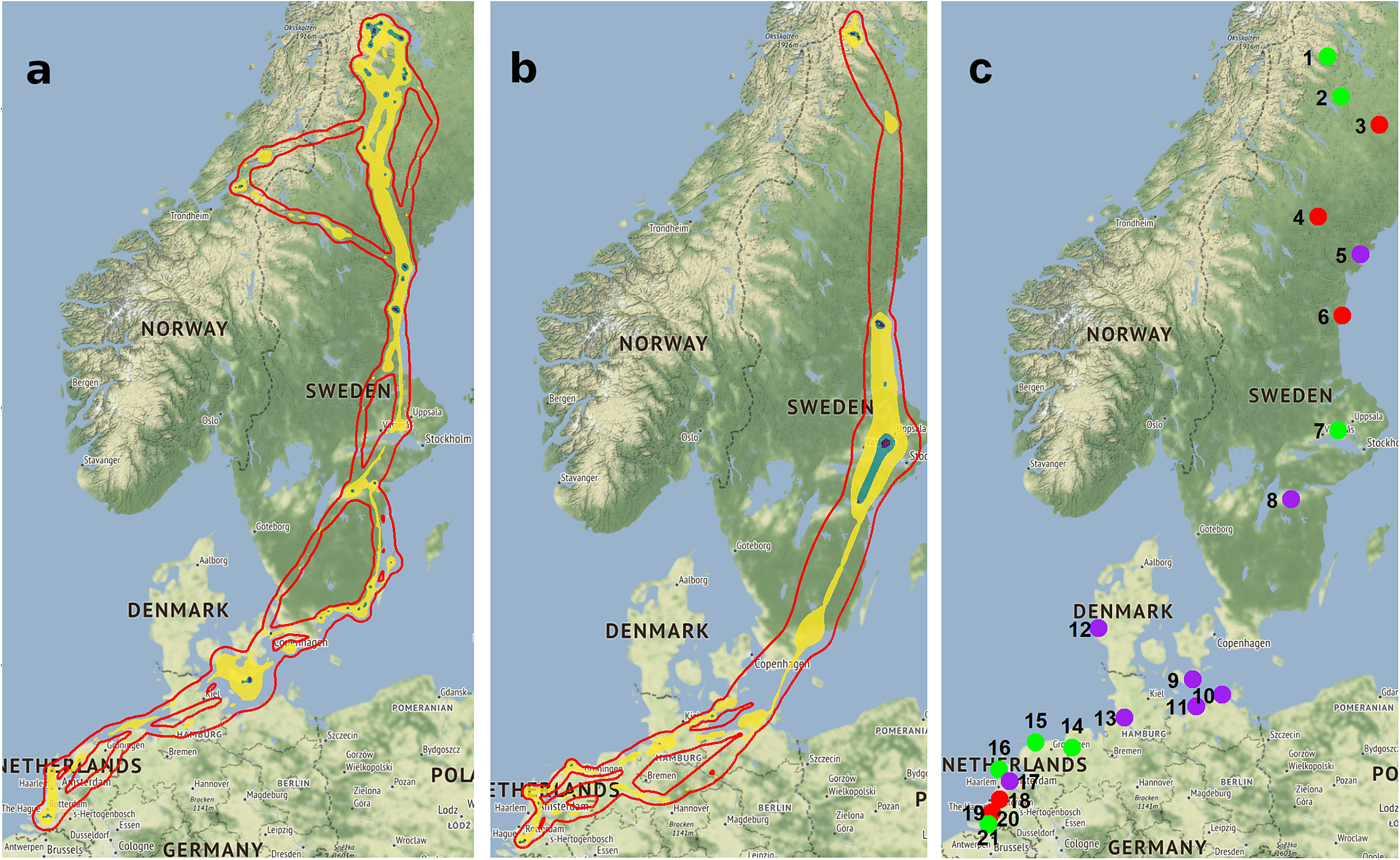
Figure 2. (a, b) Contour lines of combined dynamic Brownian bridge utility distribution volumes of all analysed GPS tracks 2015–2019. (a) Spring migration; (b) autumn migration. Colours indicate the different contours, i.e. probabilities of finding a Lesser White-fronted Goose from the Swedish population during the respective season in the enclosed/filled area: red line, 99.9%; yellow area, 99.0%; green area, 95.0%; purple area, 50%. (c) Extracted sites used by Lesser White-fronted Geese with indications of protection status: green, SPA with Lesser White-fronted Geese included; purple, SPA (partly), but Lesser White-fronted Geese not included; red, non-protected area. See names and details of sites in Table 2.
Pre-moulting flights to the coast of the Bothnian Sea (sites 5 and 6) were included in the autumn migrations. Post-moulting movements to the southern Lake Hjälstaviken (site 7) were initiated in July or the first days of August for all tracked non-breeding individuals. Interestingly, the successful breeder in 2016 (see above) arrived at the coast of the Bothnian Sea (site 5) only one day after the non-breeders and failed breeders that had moulted there (Niklas and Nina) left the area.
Autumn migration (Figure 2b) was initiated for all tracked individuals in all years by leaving Lake Hjälstaviken (site 7). From there, a single, wider migration corridor leads over south-west Sweden with only one short stopover detected at Svartåmynningen (site 8). After crossing the Baltic Sea, several birds performed short stops at various sites in Denmark, northern Germany, and the Netherlands, and four novel, albeit temporarily used, autumn stopover sites, Zingst (site 10), Langenwerder (site 11), Ringköbing fjord (site 12), and Polder Zeevang (site 17), could be identified.
In the Netherlands, we identified four sites, Driebruggen (site 18), Ridderkerk (site 19) Oudekerk (site 20), and Moerdijk (site 22), serving as alternative winter roosts to the well-known site Oude Land van Strijen (site 21) or stepping stones en route north to Petten (site 16). Furthermore, our analysis confirmed two important sites in Lower Saxony, Germany, the Lower Elbe in the district of Stade (site 13) and the Rheiderland (site 14) between the Bay of Dollart and River Ems.
Important unprotected sites during spring migration are Båtsjaur, Kaskeloukt, and Hudiksvall, where the latter is most important due to its long duration of use, also for moulting. Further non-protected sites are Driebruggen (site 18), Oudekerk (site 20), Ridderkerk (site 19), and Moerdijk (site 22) in the Netherlands that are used temporarily during winter. For five sites in protected areas, Roden Fed (site 9), Zingst (site 10), Langenwerder (site11), Ringköbing fjord (site 12), and Polder Zeevang (17), the presence of Lesser White-fronted Geese was confirmed for the first time (Table 2). Against this background, it has now been possible to clarify where the birds had been resting so far in March on their spring migration. This was previously unknown. In conclusion, of the 22 identified sites, seven (32%) are not listed as SPAs and 13 (59%) lack Lesser White-fronted Geese in the list of species prioritised (in 6 of 14 sites). An effective protection regime is important, especially with regard to threats from hunting or degradation of foraging areas.
During spring migration, the geese spent 8.5 days in unprotected areas, a total of 41.3 days in fully protected areas (SPAs with Lesser White-fronted Geese mentioned), and 51 days in SPAs where the Lesser White-fronted Goose was not a target species. During autumn migration, the birds spent a total of 10 days in unprotected areas, 3.25 days in fully protected areas, and 6.5 days in protected areas where protection measures for the Lesser White-fronted Goose had not been defined.
Discussion
We found that Lesser White-fronted Geese breeding in Sweden roughly follow the expected migration trajectory towards wintering areas in the Netherlands and Germany. A wide network of sites used by this highly threatened population was detected, including five areas until now unknown. The species is of highest priority within the European Birds Directive and member states are obliged to take protective measures and install qualified monitoring (Ssymank et al. Reference Ssymank, Hauke, Rückriem and Schröder1998). Even so, our results indicate that 32% of the sites used by Lesser White-fronted Geese are not within SPAs and in 59% of cases the species is not listed as a priority. Migration routes of Lesser White-fronted Geese breeding in Norway (Lorentsen et al. Reference Lorentsen, Øien and Aarvak1998, Aarvak and Øien Reference Aarvak and Øien2003) and Russia (Morozov et al. Reference Morozov, Aarvak and Øien2015, Reference Morozov, Aarvak and Øien2016) have earlier been studied using individual tracking revealing crucial information for conservation (previously unknown routes and wintering sites). Based on our results, we find little evidence of overlapping distribution with birds breeding in Norway. Hence, according to our data, the Swedish population may be viewed as a separate conservation unit with some weaknesses regarding protection: for example, the Lesser White-fronted Goose is not listed as a species of special concern in seven protected areas that they use for staging (cumulative average duration 57.5 days, comprising 50.5% of spring staging and 32.9% of autumn staging). Conservation measures are therefore not aligned with the needs of this species (Table 2).
Based on observer data, two major wintering sites have been described before and our data show similar spatial focus on these two sites: Oude Land of Strijen and Petten in the Netherlands (Cottaar and Brouwer Reference Cottaar and Brouwer1998). Our data show support for the suggestion that the earlier important site of Anjumer Kolken in the Netherlands is nearly abandoned by the population (Koffijberg and van der Winden Reference Koffijberg and van Winden2013). In earlier studies, based on field observations, many sites have been found to hold Lesser White-fronted Geese, albeit of unknown origin (Swedish, Norwegian, or Russian), during winter in the Netherlands (Ouweneel Reference Ouweneel1998, Reference Ouweneel2011, Koffijberg et al. Reference Koffijberg, Cottaar and van der Jeugd2005). In the last century, the general trend in winter distribution in the Netherlands has been an increasing focus of reported Lesser White-fronted Geese towards the two main sites (Koffijberg and van den Winden Reference Koffijberg and van Winden2013). Our data suggest that such conclusions may, at least partly, be biased by observation effort as our data show frequent use of other sites. The majority of such sites are found on the borders of main and, highly protected, wintering areas but also include five sites used as winter stepping stones (sites 17–20 and 22 in Table 2; cumulative duration 5.8 days) between Petten and Oudeland van Strijen. Consequently, based on this new knowledge, the delineation of already protected areas, as well as the introduction of new safe sites, may need to be considered by Dutch authorities, especially as these areas could be of great importance as alternative areas in case of disturbances in the core areas.
Prior to this study one important step during spring migration was highlighted as a fundamental gap in the knowledge of annual distribution. After flocks left the major wintering sites in the Netherlands at the end of February or in March, they remained undetected until mid-April when they reappeared on spring stopover sites in Sweden (SEPA 2011). All four transmitted birds used the same site during spring migration, that is, Roden Fed on Lolland (Denmark). After our findings, ground-based controls by local ornithologists indicated that the majority of the population use this site for several weeks on an annual basis. Consequently, this site is one of the most important areas for the species within the EU.
Following spring migration tracks to the north, data mostly confirm the known network of staging sites in Sweden, but with one important addition, namely Kaskeloukt outside Storuman in Västerbotten County (site 4 in Table 2). This region of Sweden holds few active ornithologists, which may explain the few earlier records of Lesser White-fronted Geese and highlight the need for dedicated monitoring of this site in coming years.
Most of the Lesser White-fronted Geese studied here stage in protected areas during migration. During spring migration, they spent about 10% of the cumulative staging time in unprotected areas. However, they spend most of their time in protected areas which do not provide special concerns regarding the Lesser White-fronted Goose (50.5%). During autumn migration, the use of completely unprotected areas even predominates; only 15% of the cumulative time is spent in protected areas with specific actions for protecting of the species e.g. in regards to disturbance, hunting or derogation shooting directly to other goose species. Apart from the one successful breeder, John in 2016 (see Table 1), all tracked birds left the breeding area in June to moult in Hudiksvall (site 6, Table 2) on the coast of the Bothnian Sea. Long-distance movements to moulting sites during non-breeding years or birds that failed breeding have previously been reported for the Norwegian population (Lorentsen et al. Reference Lorentsen, Øien and Aarvak1998, Øien and Aarvak Reference Øien, Aarvak and Riede2001). Interestingly, we could not detect any differences in spring or autumn migration staging of the male bird John in his failed year (2015) vs his successful year (2016). We have chosen not to show, or share, specific coordinates, or detailed information about movements close to breeding area or moulting sites to avoid potential exposure of sensitive information as the species is highly vulnerable to human disturbance (Tian et al. Reference Tian, Solovyeva, Danilov, Vartanyan, Wen, Lei, Lu, Bridgewater, Lei and Zeng2021).
We found that migration routes and strategies differ between spring and autumn for our studied birds, which agrees with findings from other goose species (Nilsson Reference Nilsson, Klaassen and Alerstam2013). Using higher numbers of sites and longer duration for spring migration compared with autumn migration have been shown, for example, for Greater White-fronted Geese (Kölzsch et al. Reference Kölzsch, Müskens, Kruckenberg, Glazov, Weinzierl, Nolet and Wikelski2016), Bar-headed Geese (Guo-Gang et al. Reference Guo-Gang, Dong-Ping, Yun-Qiu, Ming, Fa-Wen, Jun, Zhi and Feng-Shan2011), and also Lesser White-fronted Geese in Norway or the Siberian Far East (Aarvak and Øien Reference Aarvak and Øien2003, Lei et al. Reference Lei, Jia, Zuo, Zeng, Shi, Zhou and Wen2019). All our tracked birds started autumn migration from the same site (site 7), which was reached after southbound post-moult movements. In most years all active transmittered birds left this important stopover site on the same night or within three days, indicating coordinated departure of flocks from the site, typically followed by non-stop flight to the next stopover south of Sweden. Stepping-stone sites before reaching the winter areas in the Netherlands were detected mainly in northern Germany (sites 10-11 and 13-14) (Table 2), but also in Denmark (site 12). Our tracked birds used these sites mainly for short-stopping before arrival at the wintering sites. Earlier and recurrent reports of Lesser White-fronted Geese at some of these sites suggest usage by other flocks or that duration of stay can vary between years.
From a species protection perspective mapping all sites used during migration and wintering is essential for a vulnerable population like the Lesser White-fronted Goose in Western Europe. It is becoming increasingly necessary to assess the risk of birds being accidentally shot on a site-specific basis. With rapid population growth of some goose species and increasing conflicts with human interests (Buij et al. Reference Buij, Melman, Loonen and Fox2017), some stakeholders have called for increased hunting pressure on common goose species. However, Lesser White-fronted Geese using more easterly migration routes are now known to be at high risk of being accidentally killed by the hunting of other goose species (Jones et al. Reference Jones, Martin, Barov and Nagy2008), especially with regard to Greater White-fronted Geese (Anser albifrons). Due to changes in policy regarding goose-related agricultural damage, protection status has been relaxed for many goose species in Western Europe. As a consequence, in many areas along the migration route culling of Greater White-fronted Geese is allowed, either during an open season or under a legal framework allowing shooting to prevent crop damage. The “look-alike dilemma” is a common and widely acknowledged challenge for goose management and conservation within Western Europe (Madsen et al. Reference Madsen, Bunnefeld, Nagy, Griffin, du Rau, Mondain-Monval, Hearn, Czajkowski, Grauer, Merkel, Williams, Alhainen and Guillemain2015).
We find little evidence of spatial overlap with migration routes described for Lesser White-fronted Geese breeding in Norway and Russia, migrating from northern Norway via Estonia to Hungary and Greece (Aarvak and Øien Reference Aarvak and Øien2003, Morozov et al. Reference Morozov, Aarvak and Øien2015, Reference Morozov, Aarvak and Øien2016), suggesting low intermixing with other populations. Geographical isolation has been suggested to, at least partly, explain low intrinsic, genetic variation and a population-specific genetic profile of the Swedish breeding Lesser White-fronted Geese (Diez-del-Molino et al. Reference Diez-de-Molino, von Seth, Gyllenstrand, Widemo, Liljebäck, Svensson, Sjorgen-Gulve and Dalén2020). In contrast, Norwegian and Russian breeders show clear intermixing and common genetic variation, which may be explained by the regular exchange of males between the two populations (Ruokonen et al. Reference Ruokonen, Aarvak, Chesser, Lundqvist and Merilä2010). Consequently, our findings in combination with genetic differences (Diez-del-Molino et al. Reference Diez-de-Molino, von Seth, Gyllenstrand, Widemo, Liljebäck, Svensson, Sjorgen-Gulve and Dalén2020), suggests that the Swedish breeding population can be viewed as a separate conservation unit.
We concur with earlier statements that tracking data deliver vital information for conservation and management for migratory populations (Lei et al. Reference Lei, Jia, Zuo, Zeng, Shi, Zhou and Wen2019, Zhang et al. Reference Zhang, Xie, Li, Batbayar, Deng, Damba and Fox2020). In order to maintain a positive population growth rate, reinforcements with captive-bred Lesser White-fronted Geese to the Swedish breeding population need to continue as long as population recovery is restrained by high adult mortality and/or low reproductive output in the wild (Schekkerman and Koffijberg Reference Schekkerman and Koffijberg2020). Releasing captive birds to reinforce goose populations may potentially lead, at least temporally, to increased spatial variation (Meyburg et al. Reference Meyburg, Bergmanis, Langgemach, Graszynski, Hinz, Börner and Vansteelant2017, Mini et al. Reference Mini, Bachman, Cocke, Griggs, Spragens and Black2013). However migration may also change rapidly in geese independently of conservation interventions (Eichhorn et al. Reference Eichhorn, Drent, Stahl, Leito and Alerstam2009, Ramo et al. Reference Ramo, Amat, Nilsson, Schricke, Rodríguez-Alonso, Gómez-Crespo and Green2015). For example, Greylag Geese Anser anser in Sweden have dramatically changed wintering and migration traditions within generations (Månsson et al. Reference Månsson, Liljebäck, Nilsson, Olsson, Kruckenberg and Elmberg2022), and Barnacle Geese adjust wintering range in response to late changes in climate and habitat (Tombre et al. Reference Tombre, Oudman, Shimmings, Griffith and Prop2019). Consequently, our study delivers an adequate basis for site protection for one of the EU’s most endangered bird populations (BirdLife International 2022). However, considering the dynamic nature of migration routes and putative increased dispersal following continuous reinforcements, we advocate future tracking studies in this population. We expect that sites, not detected in our data, may be used by the population or become recolonised in the near future.
Acknowledgements
The project “Protection of Lesser White-fronted Geese in Lower Saxony – more knowledge for a countrywide strategy of conservation” was carried out on behalf of the Naturschutzbund Deutschland Landesverband Niedersachsen (BirdLife Germany, section Lower Saxony) and funded by the Lower Saxony Wadden Sea Foundation (NWS) and the “Bingo Umweltlotterie” Lower Saxony in cooperation with the Federal State Agency of Bird Protection of Lower Saxony. The study is a joint effort with the Swedish project “Projekt Fjällgås”, which is a collaboration between the Swedish Association of Hunting and Wildlife Management, the Foundation of Nordens Ark, and the Regional BirdLife partner of Norrbotten. The Swedish project work related to this study was funded via grants from the Swedish Environmental Protection Agencies and the County Board of Norrbotten. Thanks to Reinhard Vohwinkel for advice and assistance in catching geese and Jan Beekman for his work in downloading data from the data loggers. We thank the authorities of Hudiksvall (Sweden) and Petten (Camperduin, the Netherlands) for support in catching activities in their area of responsibility. The foundation “Stifter helfen” supported the project with an ArcGIS 10.6 licence. All catching and handling of birds was carried out according to permits issued by the Swedish Environmental Agency (NV-07000-11) and using individual tags was approved by the Animal Ethics Committee of Central Sweden (#C34/13).
Thanks to Kees Koffijberg and Johan H. Mooij for giving helpful comments on early versions of our manuscript. We would also like to thank the two reviewers for their suggestions on the manuscript.
As the Lesser White-fronted Goose is globally threatened, on the IUCN and EU Red Lists, and highly sensitive to human disturbance, detailed data covering sensitive sites cannot be made public.






