Impact statement
In an era of major global change identifying and protecting coastal nursery habitats are critically important. Nursery habitats contribute disproportionately to the final numbers of adults relative to other actual or potential juvenile habitats, from any combination of four main factors, elevated fish density, growth, survival of the juveniles, and their successful movement to adult habitats. Many of the studies that have assessed the nursery function of coastal habitats have focused on structurally complex vegetated habitats, particularly seagrass, mangroves and salt marsh with less research attention focused on the nursery function of macroalgae. Although the nursery function of macroalgae has recently been reviewed in tropical seascapes a global review has been lacking. A clearer understanding of the value of macroalgal habitats as fish nursery areas globally will allow for a more balanced use of limited financial resources for conservation, as well as pave the way for the implementation of true ecosystem-based management of coastal resources. This review collates research published on the important nursery role of macroalgae within both tropical and temperate coastal seascapes and highlights the importance of structurally complex canopy-forming algae – particularly fucoids (Sargassum spp. and Cystoseira spp.) as core nursery areas for juvenile fishes within tropical and temperate seascapes.
Fish nursery functioning in coastal habitats
Coastal habitats, especially those dominated by various plant species such as mangrove forests, salt marshes and seagrass beds are particularly important as nursery areas for marine and estuarine fish species (Whitfield Reference Whitfield2017). Other coastal habitats such as kelp forests and macroalgal meadows and reefs have been less studied from a fish nursery perspective but are also potentially important habitats for certain species (Bodkin Reference Bodkin1986; Fulton et al., Reference Fulton, Fulton, Berkstrom, Wilson, Abesamis, Bradley, Akerlund, Barrett, Bucol, Chacin, Chong-Seng, Coker, Depczynski, Eggertsen, Eggertsen, Ellis, Evans, NAJ, Hoey, Holmes, Kulbicki, PTY, PKS, van Lier, Matis, Noble, Perez-Matus, Piggott, Radford, Tano and Tinkler2020). The high productivity and structural refuge provided by all the above habitats results in a great abundance and diversity of juvenile fish being located in such areas (Hyndes et al., Reference Hyndes, Kendrick, MacArthur and Stewart2003).
The nursery role of submerged estuarine and marine plant habitats has been widely accepted but the parameters of what constitutes a coastal nursery habitat for fishes have, until relatively recently (Beck et al., Reference Beck, Heck, Able, Childers, Eggleston, Gillanders, Halpern, Hays, Hoshino, Minello, Orth, Sheridan and Weinstein2001), been poorly defined. Early fish nursery studies highlighted the high food availability and shelter provided by these habitats, as well as an abundance of juvenile fishes occupying these areas (Lenanton et al., Reference Lenanton, Robertson and Hansen1982). However, it was generally agreed that putative nurseries cannot be declared on the basis of a single factor such as the high density of juveniles in a particular habitat. Hence, over the decades, the diverse criteria for what constitutes a nursery ground have grown and become increasingly complex, making it impossible for a single study to measure all criteria simultaneously (Figure 1).
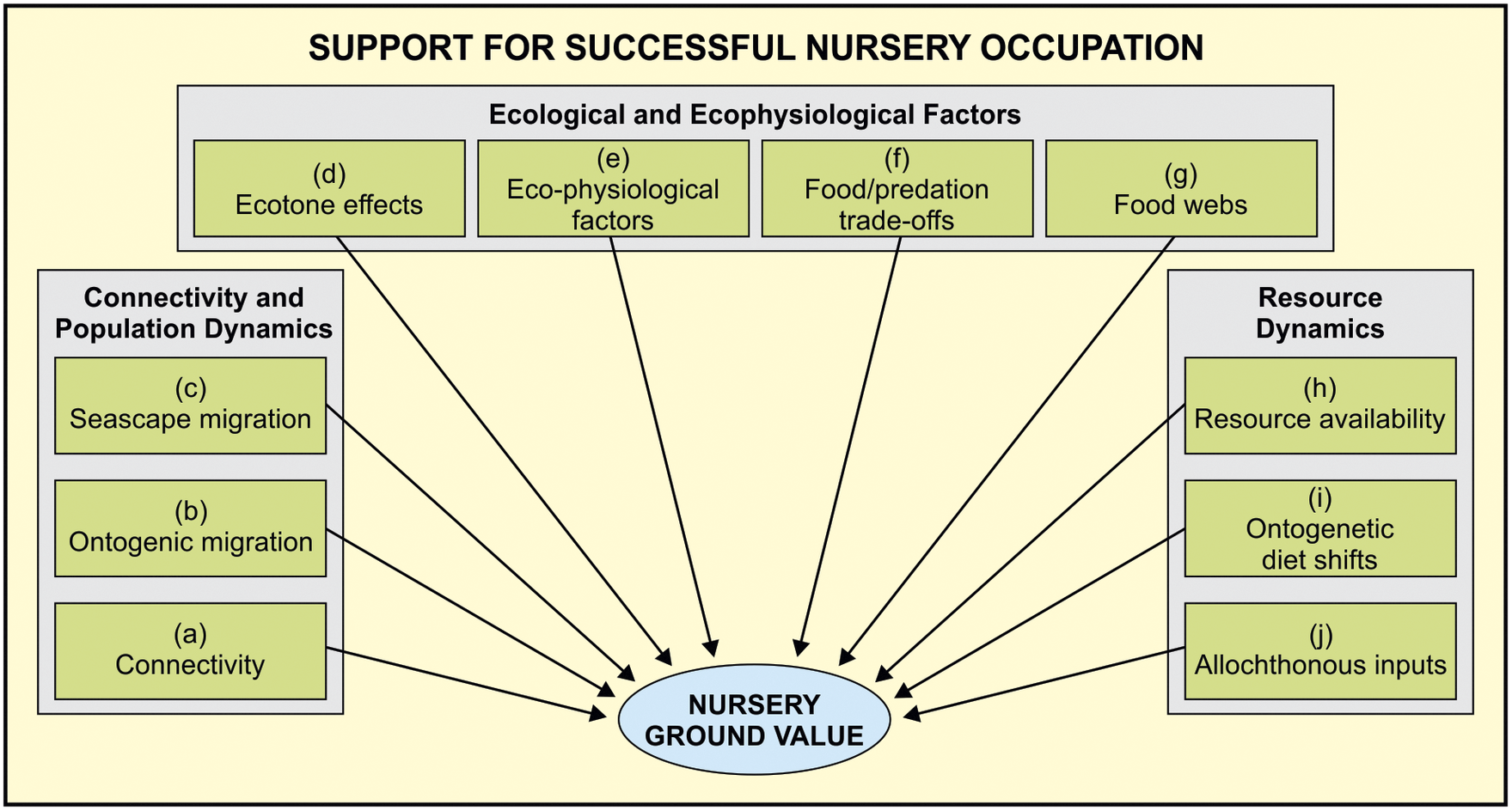
Figure 1. Components influencing fish nursery ground value in coastal ecosystems (after Sheaves et al. Reference Sheaves, Baker, Nagelkerken and Connolly2015). The true value of these ecosystems as fish nurseries is based on 10 key components grouped into three types, namely, connectivity and population dynamics, ecological and ecophysiological factors, and resource dynamics. For details on all these processes, please refer to Sheaves et al. (Reference Sheaves, Baker, Nagelkerken and Connolly2015).
Fortunately, at the turn of the century, Beck et al. (Reference Beck, Heck, Able, Childers, Eggleston, Gillanders, Halpern, Hays, Hoshino, Minello, Orth, Sheridan and Weinstein2001) put forward a relatively simple proposal that selected habitats can only be regarded as a nursery for the juveniles of a particular fish species if that habitat contributes disproportionately to the final numbers of adults relative to other actual or potential juvenile habitats. According to these authors, the disproportionate contribution to the production of adults of a particular species can come from any combination of four main factors, namely, elevated fish density, growth, survival of the juveniles, and their successful movement to adult habitats. They concluded their review by stating that researchers must compare multiple habitats when evaluating potential nursery areas, and that a particular habitat should only be considered an important nursery if it produces relatively more adults per unit area than other actual or potential juvenile habitats for a species.
There is certainly variation within and between ecosystems in the perceived value of particular plant habitats as nurseries to fish species (Bloomfield and Gillanders Reference Bloomfield and Gillanders2005), and this value also varies on a wider geographical scale (Dahlgren et al., Reference Dahlgren, Kellison, Adams, Gillanders, Kendall, Layman, Ley, Nagelkerken and Serafy2006). In this regard, researchers should not restrict themselves to determining the densities and relative abundance of juvenile fishes associated with particular habitats – they should also examine the factors or drivers that contribute to local variations in the value of a range of nursery habitats (Sheaves et al., Reference Sheaves, Baker, Nagelkerken and Connolly2015). For example, not all seagrass beds function equally as nurseries for particular fish species, and an understanding of major drivers in fish abundance in a number of localities could help explain why the nursery value of a certain habitat type is not the same on a local or regional basis (Aller et al., Reference Aller, Gullström, Maarse, Gren, Nordlund, Jiddawi and Eklöf2014). Obviously, a better understanding of the factors that create site-specific variability in nursery quality will help prioritise management efforts to halt the decline of key habitats and fish abundance in particular areas (Hughes et al., Reference Hughes, Deegan, Wyda, Weaver and Wright2002).
Although past identification and valuation of coastal marine nursery habitats for fishes typically considered habitats as individual and homogeneous entities, more recent approaches have placed a strong emphasis on critical ecological habitat linkages as defined by mobile ichthyofauna that use different habitats at different stages in their life cycle. The term ‘seascape nurseries’ was first introduced by Nagelkerken et al. (Reference Nagelkerken, Sheaves, Baker and Connelly2015) and conceptualises these nurseries as spatially explicit seascapes comprising mosaics of habitat patches that are functionally connected by the juvenile life stages of various fish species as they progress towards sexual maturity. The core area of the habitat mosaic is characterised by hotspots of juvenile fish abundance, linked to the home ranges of the occupying species. Migration pathways connect such hotspots on various spatial and temporal scales, mainly through ontogenetic habitat shifts that often result in an overall inshore–offshore migration by the various fish taxa (Mumby Reference Mumby2006).
Nursery function of macroalgal habitats
A number of coastal plant habitats, such as macroalgal beds and kelp forests, have been relatively poorly studied as potential fish nursery areas when compared to seagrass beds and mangrove forests (Whitfield Reference Whitfield2017; Lefcheck et al., Reference Lefcheck, Hughes, Johnson, Pfirrmann, Rasher, Smyth, Williams, Beck and Orth2019). The disproportionate research effort and financial resources allocated to the latter compared to the former habitats may be primarily related to research accessibility, but it may also be a function of initial subjective assessments of the relative importance of the different intertidal and shallow subtidal habitats to fishes. Nevertheless, information is emerging that these relatively poorly studied habitats may also be important core nursery areas within a mosaic of juvenile habitats, thus supporting the functionality of estuarine and marine seascapes.
In this review papers assessing the density/abundance of juveniles in macroalgal habitats and/or the role of macroalgae in providing shelter and food for juvenile fish in sub-littoral seascapes are assessed. The geographical distribution of studies reviewed is shown in Figure 2. Studies are distributed among six continents, although only one study was from Africa and three from Asia indicating the paucity of research on the nursery provision of macroalgae in these regions.
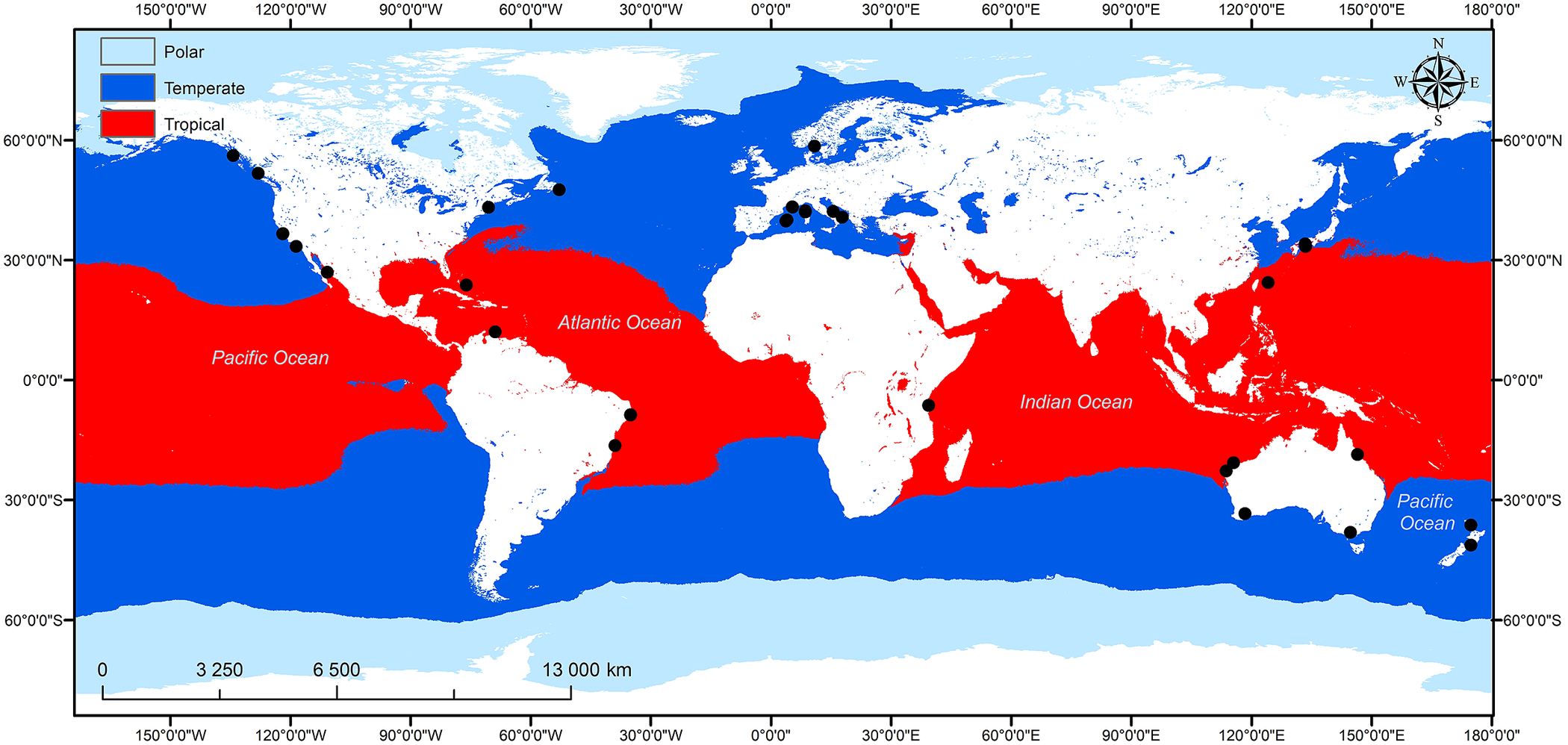
Figure 2. Global distribution of studies covered in this review that assessed the nursery function of macroalgal habitats within a nursery seascape.
Tropical macroalgae habitats
In tropical regions, a diverse mosaic of habitats is found on the leeward side of the reef crest and is collectively referred to as the back-reef system/zone (Adams et al., Reference Adams, Dahlgren, Kellison, Kendall, Layman, Ley, Nagelkerken and Serafy2006). These habitats include macroalgal fields or meadows and macroalgal clumps or patches, mangroves, seagrass beds, patch reefs, estuaries and soft and hard substrate (Adams et al., Reference Adams, Dahlgren, Kellison, Kendall, Layman, Ley, Nagelkerken and Serafy2006). Seagrass and mangroves within tropical back-reef systems are recognised as important nursery areas for many coral reef fish species, which undergo ontogenetic migrations from these vegetated habitats to coral reefs once they reach a certain size (Tano et al., Reference Tano, Eggertsen, Wikström, Berkström, Buriyo and Halling2017). Research into the nursery function of macroalgae within tropical systems has expanded considerably in recent years, with research showing that structurally complex macroalgae may perform a similar nursery function to other vegetated habitats within tropical back-reef systems (reviewed in Fulton et al., Reference Fulton, Fulton, Berkstrom, Wilson, Abesamis, Bradley, Akerlund, Barrett, Bucol, Chacin, Chong-Seng, Coker, Depczynski, Eggertsen, Eggertsen, Ellis, Evans, NAJ, Hoey, Holmes, Kulbicki, PTY, PKS, van Lier, Matis, Noble, Perez-Matus, Piggott, Radford, Tano and Tinkler2020).
Macroalgae can be found as macroalgal meadows or fields and macroalgal reef patches/clumps in tropical back-reef systems. Macroalgal meadows are defined as vast areas (>100s metres) of the seabed dominated by macroalgal communities (Evans et al., Reference Evans, Wilson, Field and Moore2014). In the tropical eastern Indian Ocean, Evans et al. (Reference Evans, Wilson, Field and Moore2014) evaluated the abundance of recruits (fish showing recruitment colouration or predetermined length) in extensive macroalgal meadows (covering approximately 71 km2) and coral reefs situated within the tropical Montebello and Barrow Islands complex. Recruits in coral reefs were dominated by species that have small bodies as adults (small-bodied zooplanktivores, corallivores, invertivores and omnivores). In contrast, macroalgal sites were dominated by species that are large-bodied as adults (emperors Lethrinidae, snappers Lutjanidae, rabbitfishes Siganidae and parrotfishes Labridae). Similarly, Wilson et al. (Reference Wilson, Depczynski, Fisher, Holmes, O’Leary and Tinkler2010) found that the composition of juvenile fish assemblages associated with macroalgal meadows and coral reefs at Ningaloo Reef in Western Australia is significantly different. Structurally complex Sargassum spp. and Dictyota spp. form extensive macroalgal meadows in the back-reef system at Ningaloo Reef. Indicative of macroalgal meadows positively affecting recruitment to adult reef populations in this region, both Wilson et al. (Reference Wilson, Depczynski, Fisher, Holmes, O’Leary and Tinkler2010) and Evans et al. (Reference Evans, Wilson, Field and Moore2014) found that a number of the species exclusively recorded as juveniles in the macroalgal meadows; including emperors Lethrinidae (Lethrinus atkinsoni), wrasse Labridae (Cheilio inermis), rabbitfishes (Siganus spp.) and goatfishes Mullidae (Parupeneus spilurus and Parupeneus barberinoides); are commonly found on coral reefs as adults. The recruitment of many of these species from nursery macroalgal meadows to coral reefs is ecologically and economically significant. For example, Siganus spp. adults perform an important ecological function on coral reefs as they are large croppers that remove macroalgae from coral reefs preventing shifts to macroalgal-dominated states. Macroalgal meadows are very important recruitment habitat for important fishery species, such as Lethrinus spp. (100% of recruits) and wrasse Choerodon spp. (82% of recruits), which are targeted by commercial and recreational fisheries in the region (Evans et al., Reference Evans, Wilson, Field and Moore2014).
Although studies from the eastern Indian Ocean highlight the importance of macroalgal meadows as nursery areas within tropical back-reef systems, relatively few studies have compared multiple macrophyte habitats within nursery seascapes. In Menai Bay (Zanzibar) in the western Indian Ocean, macroalgal beds primarily dominated by canopy-forming Turbinaria canoides and Sargassum aquifolium are found in close association with seagrass meadows dominated by Thalassodendron ciliatum (Tano et al., Reference Tano, Eggertsen, Wikström, Berkström, Buriyo and Halling2017). Within this nursery seascape, Tano et al. (Reference Tano, Eggertsen, Wikström, Berkström, Buriyo and Halling2017) compared fish assemblages between closely occurring macrophyte habitats and found a significantly higher total density of juvenile fish in macroalgal beds than in seagrass meadows. Fish assemblages were also different between the two macrophyte habitats. Juvenile wrasse and parrotfishes (Labridae) dominated the macroalgae, with juvenile moray eels Muraenidae and groupers Serranidae only found in macroalgae habitats. For these species, macroalgal beds are critically important as nursery areas. Juvenile parrotfishes Siganidae and cardinalfishes Apogonidae dominated in seagrass meadows, with fish from the family Haemulidae (grunters) only found in seagrass meadows.
In a related study assessing invertebrate resources within this seascape, Tano et al. (Reference Tano, Eggertsen, Wikström, Berkström, Buriyo and Halling2016) found that macroalgal beds had a 2.5-fold higher abundance of mobile invertebrates than seagrass meadows. Tano et al. (Reference Tano, Eggertsen, Wikström, Berkström, Buriyo and Halling2017) concluded that although both macroalgae and seagrass within this nursery seascape perform an important nursery function (in terms of provision of habitat complexity, food resources and refuge from predation) the difference in juvenile fish composition between the two macrophyte types highlights that these two habitats are not interchangeable.
In southern Brazil in the tropical southwest Atlantic, Eggertsen et al. (Reference Eggertsen, Ferreira, Fontoura, Kautsky, Gullström and Berkström2017) assessed fish assemblages associated with macroalgae (Sargassum spp.), seagrass (Halodule spp.) and reef habitats within a shallow water seascape. Sargassum is the most abundant macroalgae found along the southeast coastline of Brazil forming dense and structurally complex habitat (Chaves et al., Reference Chaves, Pereira and Feitosa2013). Similar to studies in the Indian Ocean, and indicative of core nursery function, Sargassum beds in this tropical southwest Atlantic seascape were characterised by the greatest total juvenile fish density compared with nearby seagrass and reef habitats, with juvenile parrotfishes (Scarus spp. and Scarus axillare) and surgeonfishes (Acanthurus bahianus and Acanthurus chirurgus) dominating the juvenile fish assemblage. Indicative of the importance of Sargassum as a refuge from predation, considerably fewer predators were recorded within the Sargassum beds compared to reef habitats. Similarly, Chaves et al. (Reference Chaves, Pereira and Feitosa2013) found that newly settled recruits and early juveniles, primarily wrasse, surgeonfishes, damselfishes and grunters, dominated Sargassum beds in northeastern Brazil.
In contrast, the seagrass beds present along the Brazilian coastline (Halodule wrightii) did not contain large numbers of juveniles of any species (Eggertsen et al., Reference Eggertsen, Ferreira, Fontoura, Kautsky, Gullström and Berkström2017). These findings highlight the importance of structural complexity in macrophyte habitats, as H. wrightii is low growing and patchy providing little structural complexity compared with Sargassum, which provides space for settlement as well as a number of epiphytic invertebrates that are found within their branching structure (Chaves et al., Reference Chaves, Pereira and Feitosa2013). The importance of structural complexity and macrophyte type or species to nursery provision was also demonstrated in a study by Nagelkerken et al. (Reference Nagelkerken, Dorenbosch, Verberk, Cocheret de la Morinière and van der Velde2000) in the Caribbean in the southwest Atlantic. Nagelkerken et al. (Reference Nagelkerken, Dorenbosch, Verberk, Cocheret de la Morinière and van der Velde2000) compared fish assemblages within a mosaic of back-reef habitats (including macroalgae and seagrass) and found that total juvenile density was highest in mangroves and seagrass beds (Thalassia testudinum), while macroalgal beds were not used as a nursery for juvenile reef fishes. Within this seascape macroalgal beds were dominated by low-growing, low-cover species (Halimeda spp., Caulerpa verlicillata and Cladophora spp.), which provided little shelter for juveniles fishes, whereas the dominant seagrass species T. testudinum has both high cover and canopy height. Structurally complex canopy-forming Sargassum spp. and Tubinaria spp. also dominate macroalgal reefs in the tropical Indo-Pacific where they play a vital role in the provision of nursery habitat for reef fishes (Fulton et al., Reference Fulton, Abesamis, Berkström, Depczynski, Graham, Holmes and Wilson2019). For example, in the eastern Pacific (Mexico), Aburto-Oropeza et al. (Reference Aburto-Oropeza, Sala, Paredes, Mendoza and Ballesteros2007) assessed the recruitment of leopard grouper Mycteroperca rosacea to seven different habitat types in the Gulf of California, and found the greatest number of recruits in Sargassum habitats.
Temperate macroalgae habitats
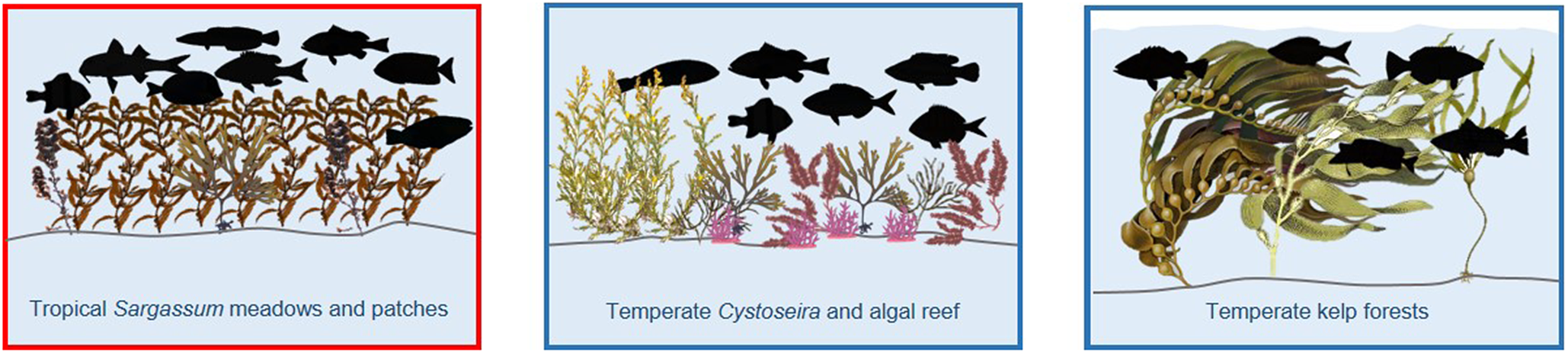
In temperate coastal seascapes, much of the three-dimensional habitat structure on rocky reefs (hard substrates) is provided by macroalgae, with this structure provided by seagrasses on soft substrates (reviewed in Thiriet et al., Reference Thiriet, Cheminée, Mangialajo, Francour, Musard, Le Dû-Blayo, Francour, Beurier, Feunteun and Talassinos2014). In cold- and warm-temperate regions, large canopy-forming brown algae dominate rocky reefs. The surface-canopy kelps (Laminariales) attain the largest size of these canopy-forming brown algae and are common in cooler regions but relatively rare along warmer coastlines. In warm-temperate regions, smaller Fucalean genera (Sargassum spp. and Cystoseira spp.) are more common. Dense beds of smaller macroalgal groups are also common in temperate areas (reviewed in Edworthy et al., Reference Edworthy, Steyn and James2022).
A lot of the work on temperate macroalgae and nursery provision for coastal fish is from the Mediterranean (see Figure 2 and Table 1). Perennial macroalgae belonging to the genus Cystoseira, which has a tree-like morphology, can form dense meadows over rocky bottoms in the Mediterranean and are referred to as Cystoseira forests (Hinz et al., Reference Hinz, Reñones, Gouraguine, Johnson and Moranta2019). These highly structured habitats potentially provide both shelter from predation and an abundance of food (with Cystoseira forests containing more prey than other less structured algal morphotypes) making them high-quality foundation-species nursery areas in addition to seagrasses in the Mediterranean (Hinz et al., Reference Hinz, Reñones, Gouraguine, Johnson and Moranta2019). Although Cystoseira forests used to dominate Mediterranean rocky reefs, these forests are being replaced by less complex Dictyotales dominated bushland, algal turfs or barren grounds as a result of numerous anthropogenic pressures, such as water pollution, invasive species, overfishing and physical disturbances (Cheminée et al., Reference Cheminée, Pastor, Bianchimani, Thiriet, Sala, Cottalorda, Lejeune and Francour2017). Dictyotales is a small ribbon-like algae and does not provide the complex three-dimensional structure of the branching Cystoseira forests (Thiriet et al., Reference Thiriet, Cheminée, Mangialajo, Francour, Musard, Le Dû-Blayo, Francour, Beurier, Feunteun and Talassinos2014). Cheminée et al. (Reference Cheminée, Sala, Pastor, Bodilis, Thiriet, Mangialajo, Cottalorda and Francour2013, Reference Cheminée, Pastor, Bianchimani, Thiriet, Sala, Cottalorda, Lejeune and Francour2017) compared juvenile fish between Cystoseira forests and less complex rocky Dictyotales spp. bushland in the northwest Mediterranean and found that juvenile fish assemblages differed between the two macroalgal habitats. Cystoseira forests had a richer and more abundant juvenile assemblage, with three-fold more abundant juvenile assemblages, and were particularly important as a nursery area for the wrasse Symphodus ocellatus, Symphodus roissali and Symphodus tinca as well as Serranus species. These differences were consistent through space at scales of 1, 10 and 40 km (Cheminée et al., Reference Cheminée, Pastor, Bianchimani, Thiriet, Sala, Cottalorda, Lejeune and Francour2017).
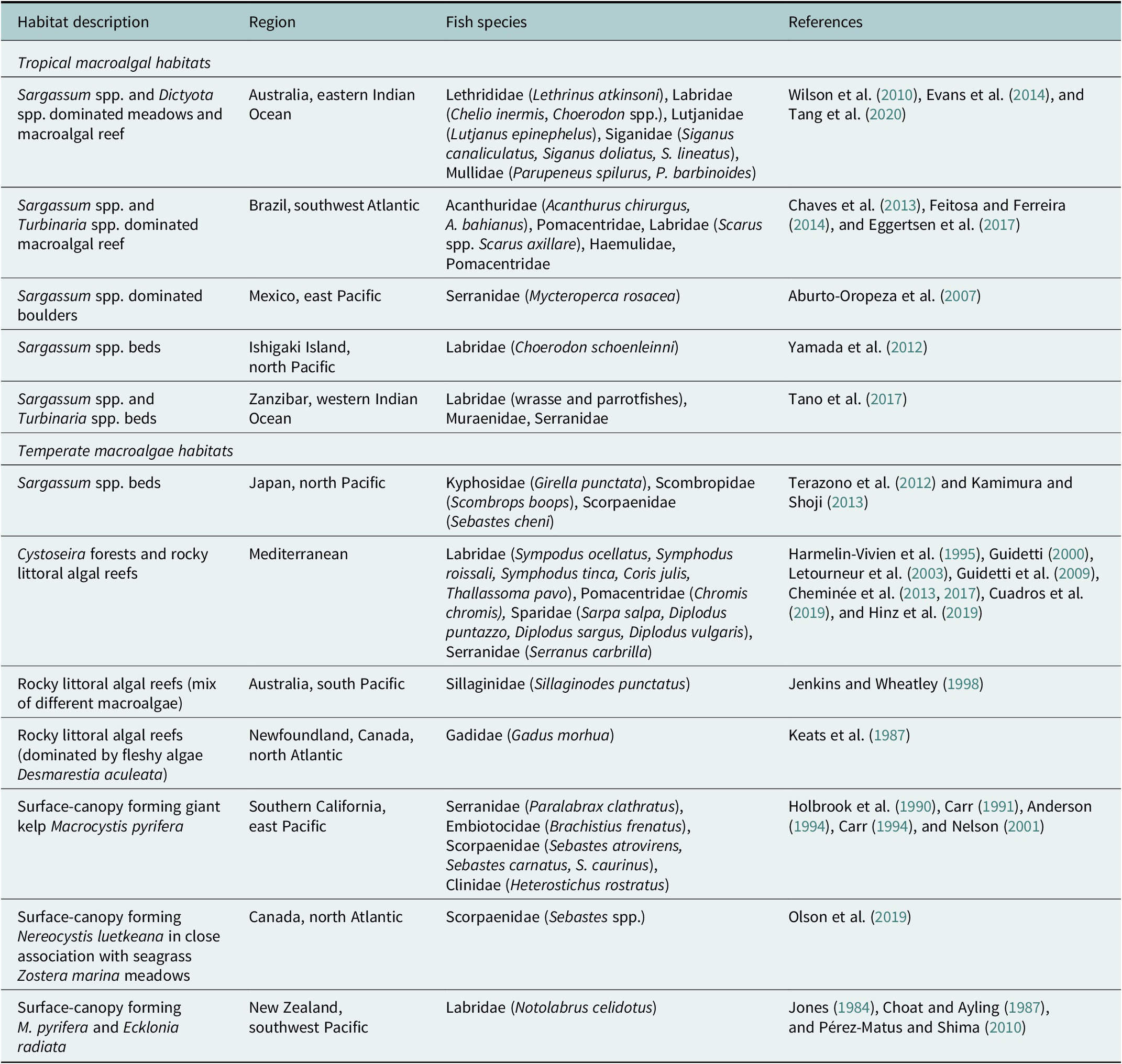
Table 1. Summary of the major macroalgal habitats reviewed their location and nursery fish species associated with each habitat
When comparing a mosaic of different rocky littoral habitats in Spain, García-Rubies and Macpherson (Reference García-Rubies and Macpherson1995) found that depth rather than substrate type was a major determinant of recruitment patterns of juvenile fish, with the smallest juveniles occurring in the shallowest depths. The exception was the goatfish Mullus surmuletus and the Labrid Symphodus cinereus, which recruited exclusively into Posidonia oceanica seagrass beds. Letourneur et al. (Reference Letourneur, Ruitton and Sartoretto2003) also found that juvenile Labrids recruited into shallow areas (<10 m depth) in southeast France, with Coris julis juveniles associated with P. oceanica and S. roissali with substrates with a high cover of fleshy algae.
In contrast, Harmelin-Vivien et al. (Reference Harmelin-Vivien, Harmelin and Leboulleux1995) found that although juvenile sparid fishes also recruit into very shallow waters (<2 m) in rocky littoral areas in southern France, substrate is also important. Juvenile Sarpa salpa, which is herbivorous, always recruited into macroalgal areas, while Diplodus annularis always recruited into P. oceanica beds. Juvenile Diplodus puntazzo, Diplodus sargus and Diplodus vulgaris preferred macroalgal habitats, although they also recruited into other habitats. The recruitment of sparids, which are often herbivorous or omnivorous, into macroalgal habitats may be influenced by the epiphytic growth on the algal fronds, as well as the ability of the fish to macerate and digest the cellular contents of the dominant macroalgae. However, considerable work still needs to be done on the selectivity of juvenile herbivorous fish for different macroalgal species, which are presumably influenced by the potential food value of algae in these nursery areas.
Within a similar mosaic of rocky littoral habitats, Guidetti et al. (Reference Guidetti, Beck, Bussotti, Ciccilella, D’Ambrosio, Lembo, Spedicato and Boero2009) assessed the nursery function of 10 different habitat types in southeast Italy. In this study, the number of settlers per habitat type was used as a proxy of juvenile fish provided to adult populations to assess (1) the Beck et al. (Reference Beck, Heck, Able, Childers, Eggleston, Gillanders, Halpern, Hays, Hoshino, Minello, Orth, Sheridan and Weinstein2001) nursery habitat criteria (NH), where a habitat is a nursery for a particular species if its contribution to adult populations is greater on average than other habitats where juveniles occur; and (2) the effective juvenile habitat (EJH) criteria (Dahlgren et al., Reference Dahlgren, Kellison, Adams, Gillanders, Kendall, Layman, Ley, Nagelkerken and Serafy2006), where a habitat is a nursery for a particular species when it contributes more than 10% of the total number of juveniles produced within the whole study area. Using this approach in terms of total settler or juvenile abundance both P. oceanica beds and sublittoral rocks covered by macroalgae were important nursery habitats within a seascape of juvenile habitats (NH and EJH). Fourteen species settled in the study area, with sublittoral rocks covered by macroalgae being an important nursery habitat (NH and EJH) for C. julis and S. roissali (Labridae), Chromis chromis (Pomacentridae) and S. salpa (Sparidae). Similarly, when comparing recruitment to rocky-algal reefs (with a dense cover of Cystoseira spp.), P. oceanica beds and bare sand at two different localities in southeast Italy, Guidetti (Reference Guidetti2000) found that C. julis occur in rocky-algal and Posidonia habitats, while C. chromis and Serranus carbrilla juveniles occur in higher densities in rocky-algal habitats. No juveniles of any species were recorded over bare sand.
In Port Phillip Bay in the temperate south Pacific (southern Australia), Jenkins and Wheatley (Reference Jenkins and Wheatley1998) assessed fish assemblages in three closely occurring habitats; unvegetated sand, macroalgal reef and seagrass (Heterozostera tasmanica). The reef habitat comprised a mix of different macroalgae (Green: Ulva, Cladophora, Caulerpa and Codium; Brown: Cystophora, Sargassum, Caulocystis, Zonaria and Ecklonia; Red: Laurencia, Centroceras, Ptilota, Heterosiphonia, Echinothamnion, Dictyomenia and Jeannerettia). Both seagrass and macroalgal reef habitats had similar fish assemblages and a significantly higher abundance of juveniles than the unvegetated sand habitat, highlighting the importance of both macroalgal reef and seagrass as nursery areas in this seascape. The pipefish Stigmatopora spp. were dominant in seagrass, whereas the King George whiting Sillaginodes punctatus preferred macroalgal reef over seagrass a month or two after settlement.
Perry et al. (Reference Perry, Staveley and Gullström2018) adopted a similar approach in the temperate Swedish Skagerrak coast (north Atlantic), comparing fish assemblages in seagrass meadows, rocky bottoms covered by macroalgae, and soft-bottom unvegetated areas. Although they gave no indication of the macroalgal communities studied, the total abundance of juveniles was significantly higher in both vegetated habitats compared to unvegetated habitats, thus indicating the importance of both seagrass and macroalgae within this shallow water seascape as core nursery areas. In rocky subtidal waters off wave-exposed eastern Newfoundland (Atlantic), Keats et al. (Reference Keats, Steele and South1987) recorded a positive relationship between the cover of fleshy macroalgae (mainly Desmarestia aculeata) and juvenile cod (Gadus morhua). Keats et al. (Reference Keats, Steele and South1987) attributed this to the importance of fleshy macroalgae as a refuge from predation, primarily by larger cod.
In temperate, sub-polar and polar rocky sublittoral areas where kelp forests occur, these large brown algae form extensive underwater habitats that cover approximately 25% of the world’s coastline (Wernberg et al., Reference Wernberg, Krumhansl, Filbee-Dexter, Pedersen and Sheppard2019). Although many ecosystem services, including nursery provision, are associated with kelp forests (Steneck et al., Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002), no studies have compared the overall density of juvenile fish (all species) in kelp with other habitat types. Key questions that need to be addressed include – are kelp forests dominated by juveniles and is the overall density of juvenile fish higher in kelp relative to other nearby habitats? Kelp, and microhabitats within kelp forests, have however shown to be important nursery areas for particular species within rocky subtidal areas. On New Zealand reefs (southwest Pacific), Jones (Reference Jones1984) and Pérez-Matus and Shima (Reference Pérez-Matus and Shima2010) found a positive association with the surface canopy forming giant kelp Macrocystis pyrifera and juveniles of the labrid Notolabrus celidotus, with juveniles only found in habitat patches with the giant kelp. Similarly, in New Zealand, Choat and Ayling (Reference Choat and Ayling1987) found that dense stands of the laminarian kelp Ecklonia radiata and fucoid Carpophyllum flexuosum in shallow water supported large numbers of juvenile N. celiodotus. Coralline reef flats supported fewer labrids, which were larger individuals, as well as larger individuals of predatory species. The association of smaller juvenile labrids with dense algal stands was restricted to shallow water (<10 m depth).
Holbrook et al. (Reference Holbrook, Carr, Schmitt and Coyer1990) found that the abundance of young-of-year kelp bass Paralabrax clathratus (Serranidae), kelp surfperch Brachyistius frenatus (Embiotocidae) and giant kelpfish Heterostichus rostratus (Clinidae) in California (temperate east Pacific) was positively related to the amount of giant kelp present on the reef, with young feeding on invertebrates associated with kelp. Carr (Reference Carr1994) also found that recruitment of P. clathratus was positively related to the amount of giant kelp present on a reef with this relationship asymptotic and peaking at intermediate densities of M. pyrifera. Similarly, Anderson (Reference Anderson1994) found that the recruitment of B. frenatus to the canopy of giant kelp was positively related to giant kelp density, with recruitment negligible below a threshold canopy density. Also in California, Nelson (Reference Nelson2001) described recruitment patterns of young-of-year kelp rockfish Sebastes atrovirens (Scorpaenidae) to different microhabitats in giant kelp. Young-of-year kelp rockfish recruited to the canopy of giant kelp, sequentially using three different microhabitats in the canopy before moving to the holdfasts on the bottom. They remain associated with the holdfasts for 3 months before moving to rocky crevices. Similarly, Carr (Reference Carr1991) found that S. atrovirens, as well as two other Sebastes species, Sebastes carnatus and S. caurinus, recruit near the surface of the giant kelp canopy. Within the canopy, settlers use algal-associated prey as well as finding a refuge from predation (Carr Reference Carr1991).
In high-latitude Alaskan waters, where the kelp Nereocystis leutkeana is the dominant canopy-forming species, the presence of Nereocystis surface canopy in summer (Nereocystis dies back in winter) was associated with a decline in shoals of juvenile cod (Gadidae), with the structure provided by Nereocystis having a negative effect on schooling behaviour of juvenile cod. In contrast, the density of juvenile benthic fishes (Pholidae, Cyclopteridae and Hemitripteridae) increased two-fold in sub-canopy algal sites with Nereocystis present compared to sites without Nereocystis. As Nereocystis canopy had no effect on prey abundance (amphipods and copepods) this increase was attributed to an indirect effect, such as a decrease in light availability in sites with surface canopy present (Siddon et al., Reference Siddon, Siddon and Stekoll2008).
Factors affecting nursery provision in tropical and temperate macroalgae habitats
Macrophyte cover/biomass, structural complexity, as well as the variety of functional forms present, play an important role in the nursery provision of different vegetated habitats. Evans et al. (Reference Evans, Wilson, Field and Moore2014) found that the percentage cover of high macroalgal canopy in tropical macroalgal meadows is an important predictor of recruit populations of Siganus spp., Lethrinus spp. and Choerodon spp. Within macroalgal meadows, they defined high-canopy macroalgae as any algae greater than 10 cm and low-canopy macroalgae as less than 10 cm. High-canopy macroalgae, with larger and longer thalli, provide more shelter from predators as well as more potential prey items. Eggertsen et al. (Reference Eggertsen, Ferreira, Fontoura, Kautsky, Gullström and Berkström2017) recorded a positive relationship between Sargassum canopy height and total juvenile fish density.
Feitosa and Ferreira (Reference Feitosa and Ferreira2014) found that higher densities of small juvenile parrotfishes were found in habitat patches dominated by Sargassum as opposed to densely packed jointed calcareous algae along the northeast coast of Brazil. This was related to protection (rather than food as food resources were available in all habitats studied); with the high canopy of Sargassum providing more shelter than densely packed calcareous algae. Similarly, Figueiredo et al. (Reference Figueiredo, Duarte and Flores2020) found that algal canopies are critical in the supply of invertebrate prey to common reef fishes along the northeast coast of Brazil compared to less structurally complex low-lying algal turfs.
Within temperate Cystoseira forests, Cuadros et al. (Reference Cuadros, Moranta, Cardona, Thiriet, Francour, Vidal, Sintes and Cheminée2019) found that although fish species composition was affected by habitat complexity (percent cover and canopy height) the total density of juveniles was not affected by habitat complexity. Among the wrasse species which use Cystoseira forests as nursery areas, Symphodus spp. favoured more structurally complex habitats, while Thalassoma pavo and C. julis preferred less complex habitats, although the smallest juveniles of T. pavo occurred in the most complex forests (Cuadros et al., Reference Cuadros, Moranta, Cardona, Thiriet, Francour, Vidal, Sintes and Cheminée2019). This pattern may be related to the trade-off between the provision of food and shelter (Cheminée et al., Reference Cheminée, Pastor, Bianchimani, Thiriet, Sala, Cottalorda, Lejeune and Francour2017). Food availability may be at a maximum for intermediate values of vegetation complexity (Grenouillet et al., Reference Grenouillet, Pont and Seip2002; Cuadros et al., Reference Cuadros, Moranta, Cardona, Thiriet, Francour, Vidal, Sintes and Cheminée2019), with larger juveniles actively selecting for less complex forests, while the smallest T. pavo and Symphodus spp. may be seeking shelter in the more structurally complex habitats (Cuadros et al., Reference Cuadros, Moranta, Cardona, Thiriet, Francour, Vidal, Sintes and Cheminée2019).
In northern New Zealand, the density of kelp had a direct impact on the recruitment of juvenile N. celidotus (Labridae), with recruitment low or non-existent in areas with low macroalgal density and absent in areas with no macroalgae (Jones Reference Jones1984). The relationship between algal biomass and recruitment was clearly demonstrated experimentally, with recruitment increasing exponentially with algal biomass in algal removal and addition experiments. This may be related to both prey availability and shelter. Juvenile N. celidotus forage among large brown algae, with the abundance of juvenile prey items increasing linearly with algal biomass. In summer, however, gammarid amphipods, which the juveniles prey on, are also available in rock-flat areas suggesting that the absence of juveniles from habitats without macroalgae may also be because of a lack of shelter (Jones Reference Jones1984).
Seascape structure and configuration, such as distance to neighbouring habitats (e.g. van Lier et al., Reference van Lier, Wilson, Depczynski, Wenger and Fulton2018; Olson et al., Reference Olson, Hessing-Lewis, Haggarty and Juanes2019; Sievers et al., Reference Sievers, McClure, Abesamis and Russ2020), composition or vegetation structure within those habitats (e.g. Levin Reference Levin1991; Hinz et al., Reference Hinz, Reñones, Gouraguine, Johnson and Moranta2019; Tang et al., Reference Tang, Graba-Landry and Hoey2020) and patch size (e.g. Deza and Anderson Reference Deza and Anderson2010) can have a significant effect on the nursery function of nursery seascapes. Focussing on a large temperate seagrass meadow, Olson et al. (Reference Olson, Hessing-Lewis, Haggarty and Juanes2019) found that recruitment of young-of-year rockfish (Sebastes spp.) was highest at the edges between seagrass (Zostera marina) and kelp forests (Nereocystis luetkeana) as well as in adjacent kelp forests. Within this seascape, the edges occurring between these two vegetated habitats were identified as optimal nursery areas (as edges provided both structural complexity and a variety of growth forms). van Lier et al. (Reference van Lier, Wilson, Depczynski, Wenger and Fulton2018) determined that macroalgal meadows within a tropical reef ecosystem influenced the diversity and abundance of Labridae across the seascape, especially when the meadows were within 500 m of the coral reef. Similarly, Sievers et al. (Reference Sievers, McClure, Abesamis and Russ2020) found that macroalgal beds near to coral reefs positively influenced wrasse densities on coral reefs. In other words, seascape connectivity can be interrupted by physical ‘gaps’ and this influences the colonisation of final marine habitats by certain fish species.
Macroalgal species assemblage (vegetation structure) in habitat patches may also influence nursery function. Macroalgal habitats in both tropical and temperate reefs comprising several functional forms (a mixture of canopy and understory taxa) have the potential to support higher juvenile diversity and abundance rather than a single homogeneous habitat type covering the same area; with this linked to both shelter (protection from predation) and food provision (Wilson et al., Reference Wilson, Depczynski, Fisher, Holmes, O’Leary and Tinkler2010; Cheminée et al., Reference Cheminée, Pastor, Bianchimani, Thiriet, Sala, Cottalorda, Lejeune and Francour2017; Tano et al., Reference Tano, Eggertsen, Wikström, Berkström, Buriyo and Halling2017). Tang et al. (Reference Tang, Graba-Landry and Hoey2020) found support for this hypothesis, with the abundance of recently-settled rabbitfishes in tropical Sargassum beds increasing with both Sargassum height and the cover of other algal morphotypes. In temperate reefs, Hinz et al. (Reference Hinz, Reñones, Gouraguine, Johnson and Moranta2019) suggested that several algal species might fulfil similar nursery functions, so that when one species is in a state of seasonal dieback another may perform a similar function. Similarly, Levin (Reference Levin1991) found that settlement-stage Tautogolabrus adsperus (Labridae) were associated with filamentous and foliose algae in coralline-dominated reefs and with tall kelp in kelp-dominated reefs. In other words, structurally complex algae in coralline-dominated reef provided similar nursery habitat to taller kelp in nearby kelp-dominated reef.
Seasonal cycles in the phenology of canopy-forming macroalgae (e.g. Sargassum) are important to consider in tropical (e.g. Fulton et al., Reference Fulton, Abesamis, Berkström, Depczynski, Graham, Holmes and Wilson2019) and temperate seascapes. During high canopy states, macroalgae provide nursery areas for new recruits, which then show ontogenetic migrations to either other nursery habitats or coral reefs (as sub-adults). In the low-canopy state, nursery function may be limited, for example, Ornellas and Coutinho (Reference Ornellas and Coutinho1998) found that the abundance of juveniles within a Brazilian tropical seascape was highest in summer, corresponding with high Sargassum algal biomass, and decreased in other seasons when algal biomass decreases. Large patches of detached macroalgae can however also serve an important nursery function for 0+ fish (Lenanton et al., Reference Lenanton, Robertson and Hansen1982).
The peak recruitment of many species within Sargassum dominated seascapes are potentially timed to coincide with high macroalgae biomass and prey availability. For example, Yamada et al. (Reference Yamada, Nanami, Ohta, Fukuoka, Sato, Kobayashi, Hirai, Chimura, Akita and Kawabata2012) found that the recruitment patterns of black-spot tuskfish Choerodon schoenleinni at Ishigaki Island (tropical Japanese waters) are spatially and temporally tied to the availability of Sargassum and prey organisms situated within the thalli. Choerodon schoenleinni initially settles within the thalli of Sargassum and other small algae before moving to seagrass as larger juveniles. Similarly, in southwestern temperate Japan juvenile Japanese rockfish Sebastes cheni recruit first to Sargassum beds before migrating to seagrass (Zostera spp.) beds as larger juveniles (Kamimura and Shoji Reference Kamimura, Shoji, Moksness, Dahl and Stottrup2013). On rocky reefs in southwestern Japan, temperate, perennial Sargassum species are the main canopy-forming species and provide important nursery habitats for many fish species (Terazono et al., Reference Terazono, Nakamura, Imoto and Hiraoka2012). Temperate Sargassum species are being replaced by expanding tropical Sargassum species, and although both are used by the same fish assemblages, the shorter vegetation period of tropical Sargassum may ultimately affect the recruitment of temperate fish species to these nursery areas (Terazono et al., Reference Terazono, Nakamura, Imoto and Hiraoka2012).
Summary
Coastal management authorities commonly consider salt marshes, mangroves and seagrass beds as valid fish nursery areas, with less attention given to macroalgal habitats as coastal nursery habitats for both fishes and invertebrates. This lack of information will hinder the protection of macroalgal meadows and macroalgal reefs, which, in an era of major global change is certainly cause for concern. Thus, a clearer understanding of the value of macroalgal habitats as fish nursery areas in particular will allow for a more balanced use of limited financial resources for conservation, as well as pave the way for the implementation of true ecosystem-based management of coastal resources.
This review has highlighted the importance of smaller canopy-forming brown algae from the Fucalean genera (Sargassum spp.) as core nursery areas for juvenile fishes, particularly emperors (Lethrinidae), rabbitfishes (Siganidae), wrasse and parrotfishes (Labridae), goatfishes (Mullidae), groupers (Serranidae), surgeonfish (Acanthuridae) and damselfish (Pomacentridae) within tropical back-reef systems. Similarly, in temperate nursery seascapes, the importance of Fucalean genera (Cystoseira spp.) and macroalgae reefs as core nursery habitats for damselfish (C. chromis), groupers and numerous species of wrasse and sparids was highlighted. Although the overall density of juvenile fish was not shown to be higher in the larger, surface canopy-forming kelp relative to other temperate nursery habitats, the giant kelp was important in the recruitment of Notolabrus celiodotus (wrasse), Paralabrax clathrus (Serranidae), B. frenatus (Embiotocidae), H. rostratus (Clinidae) and Sebastes spp. (Scorpaenidae). When macroalgae and seagrass were compared, the nursery function of structurally complex macroalgae, in terms of the density of recruits and juveniles, was found to be similar to that of seagrass in both temperate and tropical seascapes. These two macrophyte habitats are not however interchangeable as the species assemblage was mostly different between the two.
Although much of the research covered by this review has focused on the nursery provision of canopy-forming brown algae in both tropical and temperate seascapes, studies have highlighted that several algal species may perform similar nursery functions. In the light of global change-induced shifts in the composition and canopy structure of macroalgal meadows and reefs (Fulton et al., Reference Fulton, Abesamis, Berkström, Depczynski, Graham, Holmes and Wilson2019), it is vital that research attention also focuses on the nursery provision of other canopy-forming macroalgae, for example, red algae Plocamium and Gelidium spp. and different algal morphotypes. It is also important that more studies are conducted on seasonal changes in habitat quality and the relative importance of macroalgal beds as providers of both food (by focussing on prey availability and juvenile feeding) and shelter, similar to the nursery value approach adopted by Hinz et al. (Reference Hinz, Reñones, Gouraguine, Johnson and Moranta2019). At the seascape level, research needs to focus on the connectivity, composition and configuration of various juvenile habitat types.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/cft.2022.3.
Acknowledgements
We are grateful to Dr Paul-Pierre Steyn for expert advice on coastal macroalgae and for reading through our manuscript.
Author contributions
N.C.J. conceptualised and wrote sections of the review. A.K.W. wrote sections of the review. Both authors edited the final draft.
Financial support
This review forms part of a broader research project on South African nursery seascapes funded by the National Research Foundation (NRF) coastal and marine research grants (grant number 136489).
Competing interests
The authors declare no competing interests exist.






Comments
Herewith please find our manuscript entitled “The role of macroalgae as nursery areas for fish species within coastal seascapes” by Nicola James and Alan Whitfield for consideration in Coastal Futures. This review collates research published on the important nursery role of macroalgae within both tropical and temperate coastal seascapes.