INTRODUCTION
Epstein–Barr virus (EBV) is a ubiquitous herpesvirus infection that persists indefinitely in the host. It is transmitted via close oral contact in the setting of childhood crowding, in which infection is common in young children and is typically asymptomatic [Reference Bannister, Bannister, Begg and Gillespie1]. Infectious mononucleosis (IM) is a clinical manifestation of primary EBV infection that occurs chiefly in adolescents and young adults who escaped infection as children [Reference Bannister, Bannister, Begg and Gillespie1].
Even when primary EBV infection occurs relatively late, symptomatic IM occurs in only a fraction of primary infections [Reference Evans, Niederman and Evans2]. Prospective serological studies in college and military academy students in the USA, Scotland and Hong Kong show a consistent 12–13% seroconversion rate to EBV positivity in the first year of college, but the incidence of IM in the seroconverted varies greatly between institutions (range 28–74%), implying the existence of important risk factors other than age [Reference Sawyer3–Reference Dan and Chang7].
The extent to which genetic factors play a role in host response to initial infection, seroconversion, and manifestation of clinical symptoms of IM has been examined in a limited number of studies [Reference McAulay8–Reference Helminen11]. Some have reported that polymorphisms in genes coding for HLA [Reference McAulay8] and cytokines [Reference Hurme and Helminen9–Reference Helminen11] are associated with an increased risk of IM. However, none of these studies had enough power to adjust for other potential confounding factors.
Because identical (monozygotic; MZ) twins and fraternal (dizygotic; DZ) twins share 100% or, on average, 50% of the genotype, respectively, a comparison of twin concordance by zygosity can be used as a crude indicator of heritable susceptibility [Reference Martin, Boomsma and Machin12]. In addition, twins are matched on early childhood environment, important because early childhood EBV infection may mitigate risk of later IM. For this reason we sought to investigate the heritability of IM in twin pairs identified from a population-based registry of California-born twins.
METHODS
This study was conducted according to the guidelines set by the Declaration of Helsinki, and was completed without reference to any data that might personally identify the selected subjects. Approval for the study was obtained from the University of Southern California Institutional Review Board.
Source of subjects and data instruments
The California Twin Program (CTP) is a population-based registry of twins born in California between 1908 and 1982, and described in detail elsewhere [Reference Cockburn13]. Briefly, twins were identified from California birth records and contact addresses were obtained by linkage with records of the California Department of Motor Vehicles. From 1991 to 2001, a 16-page screening questionnaire was sent to the 115 733 individual twins with verifiable addresses; 51 609 individual twins returned the completed questionnaire yielding a response rate similar to or better than those reported in similarly aged persons in other cohort studies [Reference Bernstein14, Reference Kolonel15]. This study is based on the subset of 6926 double-respondent twin pairs born from 1957 to 1982 who received an updated questionnaire requesting more detailed information on medical conditions.
Each twin was asked whether either the respondent or their co-twin ever had one of several infectious diseases including IM, and if so, to indicate the age at onset. Information on zygosity, gender, race/ethnicity and education was also obtained. The twin pair constituted the unit of analysis, and the study was restricted to those twin pairs whose members agreed on their zygosity. Study variables included zygosity (MZ, DZ), sex of the pair (male-male, female-female, opposite-sex), race/ethnicity (White, Latino, African-American, Native American, Asian, Other), education level (less than college graduate, equal to or more than college graduate) and age at onset (continuous).
Statistical analysis
To determine the reliability of the proxy report from a twin about their co-twin, we assessed the percent agreement and the kappa statistic of the self-reported and proxy-reported occurrence of IM from the members of double-respondent pairs. The kappa statistic showed that the agreement between self and proxy reports was higher than predicted by chance (P<0·0001) but the magnitude was low (kappa=0·25–0·28). Although inclusion of proxy respondents produced similar results, we judged proxy reports to be insufficiently reliable and conservatively limited the formal analysis to self-reported IM from double-respondent pairs. Pairs from whom only one member reported IM were designated ‘discordant’, and those from which both members reported the same condition were designated ‘concordant’.
Pairwise concordance estimating the probability of disease occurring in both members of a pair given that one twin was affected with the disease was calculated as follows:
Asymptotic variance and standard errors (s.e.) were calculated using methods suggested by Witte et al. [Reference Witte, Carlin and Hopper16].
DZ twinning is associated with maternal age and parity [Reference Bonnelykke17, Reference Hoekstra18], and because large California families tend to be of lower socioeconomic status, it is a potential confounder. Pairwise concordance was reported separately by education level and sex. Because over 90% of the study population was white, we did not stratify by race.
The Cox proportional hazard function and survival curve modelling (χ2 test using phreg and lifetest procedures) were used to determine the rate of developing IM in the initially unaffected co-twin, conditioned on the age of diagnosis in the first diagnosed (index) case-twin, in MZ relative to DZ co-twins (hazard ratio). The interval of risk was defined as the time from the age of disease onset in the index case-twin to the onset of disease in the co-twin or the time of questionnaire completion (total 8257·3 person-years). We also evaluated whether the age of the index twin's onset of disease modified the risk to the co-twin. Age at disease onset was available for 87% of twins (n=585). Because males and females may have different social behaviour and therefore interpersonal contact and exposure, we repeated the analysis using only the same-sex twin pairs.
Statistical analyses were performed using SAS 9.1 (SAS Institute, USA) and 95% confidence intervals (CI) and/or P values have been reported when applicable.
RESULTS
Of the 6926 double-respondent twin pairs who completed the updated version of the CTP questionnaire, at least one member of 669 pairs reported IM (Table 1). Twins were slightly more likely to be white but were otherwise demographically similar to the total twin population. Age at completion of the questionnaire ranged from 18 to 44 years and was similar in twins reporting IM and the total double respondent CTP twin population. With the exception of zygosity, IM-concordant and IM-discordant twin pairs were similar demographically.
Table 1. Demographic information for California twin pairs born in 1957–1982
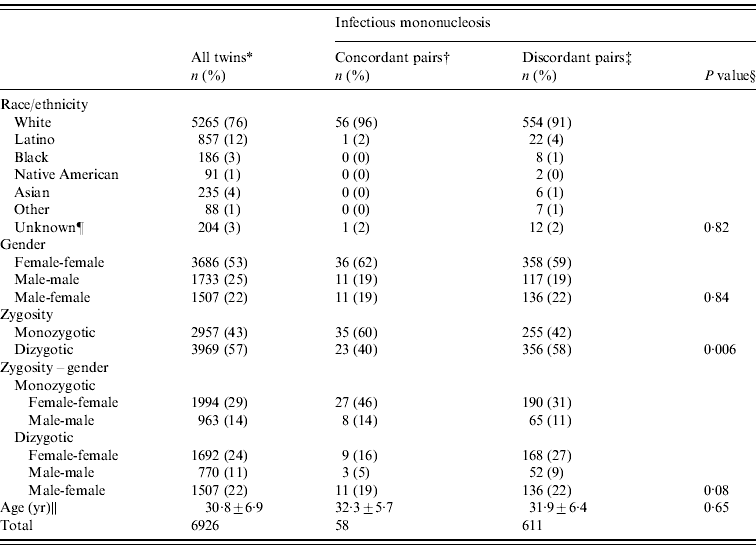
* All twin pairs in whom both members completed the screening questionnaire.
† Both members of the pair were diagnosed.
‡ One member of the pair was diagnosed.
§ P values are based on χ2 test for the categorical variables and based on two-sample t test for the continuous variables.
¶ Twin pairs in which members' responses did not agree with each other.
|| Mean age at completion of the questionnaire ±standard deviation.
Pairwise concordance for IM was twice as high in MZ compared to DZ twins (Table 2, concordance ratio 2·0, 95% CI 1·2–3·3), and the difference was statistically significant (difference in concordance=6%, s.e. of difference=2%, P χ2=0·008). The higher concordance in MZ twins persisted when stratified by gender and education. Differences were stronger when compared to same-sex DZ twins (concordance ratio 2·3, 95% CI 1·2–4·4, difference in concordance=7%, s.e. of difference=3%, P χ2=0·008).
Table 2. Pairwise concordance for infectious mononucleosis
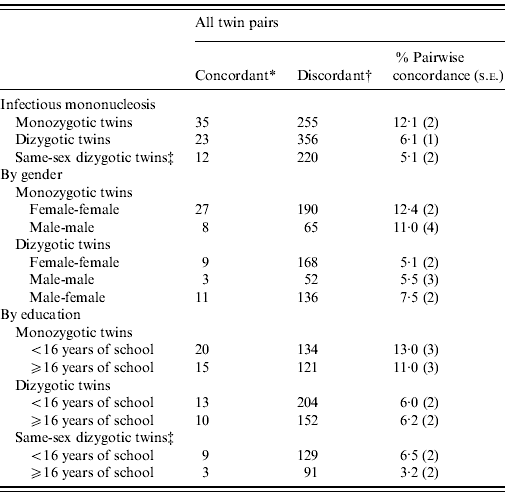
* Both members of the pair were diagnosed with infectious mononucleosis.
† One member of the pair was diagnosed with infectious mononucleosis.
‡ Subset of dizygotic twins who are male-male or female-female pairs.
Age at data collection was normally distributed (data not shown). The overall mean age at onset for IM was 17±5 years. Generally, the age at onset for cases in discordant pairs was similar to the average of cases in concordant pairs (data not shown).
MZ co-twins of cases remained at consistently higher risk of IM over time relative to DZ twins (hazard ratio 1·9, 95% CI 1·1–3·4, P=0·03) (Fig. 1). MZ co-twins had an even higher risk compared to same-sex DZ co-twins (hazard ratio 2·5, 95% CI 1·2–5·3, P=0·02). Age at IM onset in the index case did not modify risk to the co-twin (P interaction=0·7, data not shown).
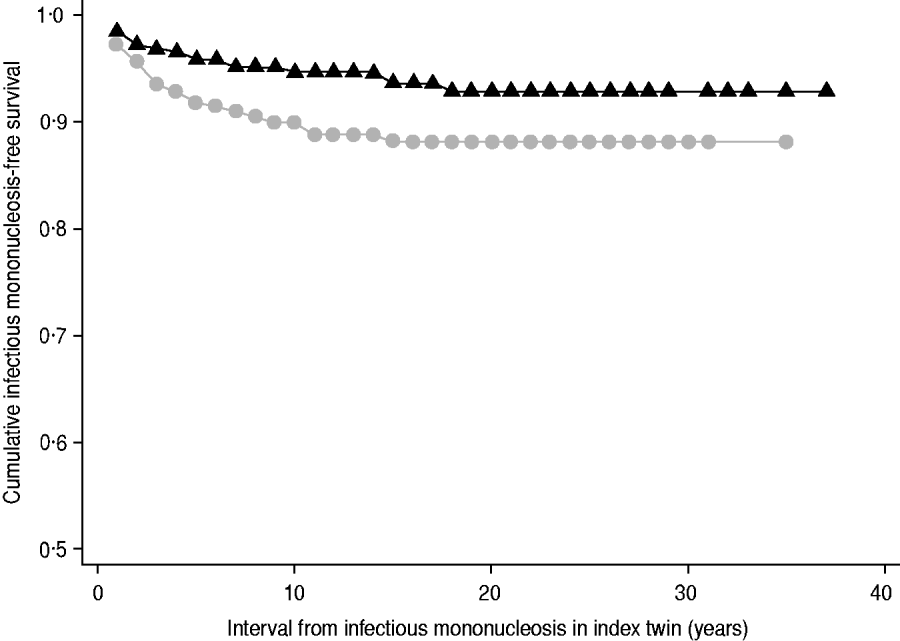
Fig. 1. Kaplan–Meier curve of freedom from infectious mononucleosis in co-twins of index cases by zygosity. ![]() , Dizygotic,
, Dizygotic, ![]() , monozygotic.
, monozygotic.
DISCUSSION
Concordance for IM in MZ twins was twice that of DZ twins when considered together and separately by sex and education level. Because age at exposure to EBV is a determinant of IM, it is possible that the higher MZ concordance could be due to more shared exposures in early childhood and adolescence in MZ compared to DZ twins. Exposure to infections transmitted by the oral or respiratory route requires person-to-person contact, and therefore the behavior of twins, parents and others plays a role. Critiques of twin studies have argued that MZ twins are treated more similarly by parents and peers, and thus have more similar early childhood exposures compared to DZ twins, which could contribute to higher trait concordance in MZ compared to DZ twins [Reference Evans and Martin19–Reference Kendler22]. However, because IM concordance in MZ pairs was double that of DZ pairs, and because MZ twins share on average twice as many genetic characteristics as DZ twins, a genetic contribution to IM aetiology is also a plausible explanation [Reference Evans and Martin19, Reference Eaves, Foley and Silberg20]. If concordance was based mainly on environmental factors (e.g. time of exposure, number of potential contacts), we might expect concordance to differ by sex, because male and female twin pairs have different levels of shared behaviour, including interpersonal contact, and therefore exposure [Reference Hamilton23]. Instead, we observed that the twofold higher concordance for MZ twin pairs was similar for both male and female pairs, consistent with an underlying sex-independent genetic susceptibility. The higher concordance in opposite-sex vs. same-sex DZ pairs is probably a reflection of chance variation.
The seroconversion rate during college age is consistent across surveyed groups, but the IM attack rate among seroconverters is variable [Reference Sawyer3–Reference Crawford6], indicating that the rate of exposure to EBV remains consistent, but the rate of IM in the exposed shows individual variation. Crawford and colleagues have postulated that multiple exposures to sources of infection could be a risk factor for developing IM [Reference Crawford24]. Another possible, but not mutually exclusive, explanation is that heritable factors play a role in host response and susceptibility.
Results from several studies of genetic susceptibility to primary EBV infection suggest that a heritable factor might produce differences in antigen recognition, immune response or the magnitude of cytokine production in response to infection [Reference McAulay8–Reference Helminen11]. The target cell of primary EBV infection is B cells and the symptoms of IM are a result of immune response to the EBV infection, including excessive production of inflammatory cytokines by T cells [Reference Farrell25]. A genetic contribution to the variability in cytokine secretion in response to antigens has been demonstrated, this could also partially explain a heritable IM susceptibility [Reference de Craen26]. Polymorphisms in genes encoding IL1-β and IL1 receptor antagonist (IL-1Rα) has been associated with EBV seropositivity and IM severity in children [Reference Hurme and Helminen9]. A haplotype in the IL10 promoter region has been linked to increased IL-10 levels in blood and protection against primary EBV infection and IM [Reference Helminen11]. IL-10 is an anti-inflammatory cytokine that is also involved in cytotoxic T cell differentiation and induction [Reference Hurme10, Reference Helminen11]. Variation in HLA epitopes could result in differential binding and activation of cytotoxic T lymphocytes against the lytic viral epitopes contributing to clinical disease [Reference McAulay8, Reference Hislop27]. HLA class I polymorphisms are associated with EBV-positive Hodgkin's lymphoma [Reference Diepstra28, Reference Hjalgrim29], and McAulay et al. found that these same polymorphisms are present more frequently in IM patients than in EBV-seronegative or asymptomatic EBV-seropositive individuals [Reference McAulay8]. These findings suggest that genetic variation in antigen recognition and cytokine production may be important in IM susceptibility and support a biologically plausible mechanism for heritability.
One limitation of this study is reliance on self-reported disease status and zygosity. The validity of self-reported IM has been assessed several times with good results. Crawford and colleagues reported over 90% accuracy of self-reported IM compared to medical records of college students [Reference Crawford24]. Self-reported zygosity is more than 95% predictive of molecular zygosity, based on studies including subjects from the present source population [Reference Cockburn13, Reference Jackson30]. Even if present, a minor non-differential misclassification of zygosity would slightly dampen, and not increase, the estimated concordance difference.
The unaffected co-twin of any recognized case might be scrutinized more carefully at time of presentation of the index case and thus produce a diagnostic bias, i.e. over-reporting of the IM in the second twin, which could lead to overestimation of concordance. However, such a bias is unlikely to vary much by zygosity.
We restricted our analysis to double respondent twins with disease status ascertained by self-report from each member of the pair. As expected, a higher proportion of MZ than DZ pairs responded in tandem. Although concordance is usually the most important motivating factor for twin participation, in this situation, response is not likely to be related to IM concordance because IM was one of many exposures queried and not the main focus of the questionnaire. Therefore, although a lower response rate for DZ (compared to MZ) twins in the CTP is likely, we would not expect it to affect the proportion of zygosity-specific IM concordance.
Twins in the CTP have been shown to be representative of the general California population with respect to age, sex, race and residential distribution [Reference Cockburn13]. The average age at IM diagnosis in both MZ and DZ twin cases is similar to that in IM cases in the general population [Reference Heath31].
Our results provide suggestive evidence for a genetic contribution to IM. Because IM has been linked to chronic conditions, such as EBV-positive Hodgkin's lymphoma [Reference Hjalgrim32] and multiple sclerosis [Reference Thacker33], understanding the nature of this genetic susceptibility may provide valuable clues to the aetiology of these and other important chronic diseases.
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (CA110836, CA58839, P30AG017265) and the California Tobacco-Related Disease Research Program (7RT-0134H, 8RT-0107H, 6RT-0354H).
The authors thank Alicia Nelson for her administrative assistance, and the twins for their continued participation in the California Twin Program.
DECLARATION OF INTEREST
None.





