Introduction
The pandemic of the coronavirus disease 2019 (COVID-19, caused by SARS-CoV-2) may impact worldwide mental health (Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020). Initial studies from China, where the epidemic started in late-2019, reported high rates of depression, anxiety, and stress symptoms in quarantined communities. These findings were reported not only in COVID-19 patients (Zhang et al., Reference Zhang, Lu, Zeng, Zhang, Du, Jiang and Du2020), but also in psychiatric samples (Zhou, Liu, Xue, Yang, & Tang, Reference Zhou, Liu, Xue, Yang and Tang2020), health care workers (Huang & Zhao, Reference Huang and Zhao2020), and the general population (Gao et al., Reference Gao, Zheng, Jia, Chen, Mao, Chen and Dai2020). A recent systematic review (Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020) showed high rates of symptoms of depression (14.6% to 48.3%), and anxiety (6.33% to 50.9%) in populations during the pandemic.
However, although these studies raised important concerns on the surge of, possibly, the ‘next global pandemic’ (de Jesus Mari & Oquendo, Reference de Jesus Mari and Oquendo2020), assessments conducted worldwide were mostly focused on symptoms, and not on diagnoses. Moreover, the studies employed convenience, online samples with no prior information of participants’ mental status (Vindegaard & Benros, Reference Vindegaard and Benros2020; Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020). Thus, due to the absence of longitudinal data, changes compared to pre-pandemic levels were not assessed.
Another issue is that the mental impact of the pandemic in communities from low- and middle-income countries has not been addressed. For instance, female sex, income, educational level, psychiatric comorbidities, and worse physical health have been associated with unfavorable mental health outcomes in these countries (Alonso et al., Reference Alonso, Mortier, Auerbach, Bruffaerts, Vilagut and Cuijpers2018; Musliner, Munk-Olsen, Eaton, & Zandi, Reference Musliner, Munk-Olsen, Eaton and Zandi2016) and might be risk factors in the pandemic. Also, studies in China and developed countries explored whether age, severity of lockdown and disruption of daily activities, physical distancing, chronic diseases, and worries associated with contracting or having severe presentations of COVID-19 were associated with mental outcomes, with mixed findings (Pierce et al., Reference Pierce, Hope, Ford, Hatch, Hotopf, John and Abel2020; Prati & Mancini, Reference Prati and Mancini2021).
Therefore, there is mixed evidence of worsening psychopathology during the pandemic. Thus, we further examined pandemic-related changes in mental symptoms and diagnoses, and their determinants, in the Brazilian Longitudinal Study of Health (ELSA-Brasil), a well-characterized Latin American cohort. Our aims were threefold:
(1) to compare the rates of psychiatric disorders and changes in symptomatology between pre-pandemic and pandemic assessments. We hypothesized that an increase in psychiatric diagnoses and symptoms would be observed, as previously reported (Vindegaard & Benros, Reference Vindegaard and Benros2020);
(2) to assess the overall changes of psychiatric symptomatology during intra-pandemic assessments; similarly, we hypothesized that psychiatric symptoms would increase, according to the literature (Salari & Hosseinian-Far, Reference Salari and Hosseinian-Far2020);
(3) to assess whether variables such as age, sex, ethnicity, educational level, clinical and psychiatric comorbidities, exposure to COVID-19, adherence and agreement to physical distancing and other quarantine measures, leisure activities, employment status, and financial impact, would be associated with mental disorders. We hypothesized that female sex, lower educational level, non-white ethnicity, and psychiatric comorbidities would be risk factors for mental disorders, as observed in earlier ELSA-Brasil studies (Brunoni et al., Reference Brunoni, Santos, Passos, Goulart, Koyanagi, Carvalho and Benseñor2019; Librenza-Garcia et al., Reference Librenza-Garcia, Passos, Feiten, Lotufo, Goulart, de Souza Santos and Brunoni2020). We also hypothesized that the elderly, people with low physical health, and those with risk factors for severe forms of COVID-19 would be more stressed and thus being at greater risk for developing mental disorders. Moreover, we expected that variables associated with loneliness and stress would be associated with greater mental disorder risk (Killgore, Cloonan, Taylor, & Dailey, Reference Killgore, Cloonan, Taylor and Dailey2020), and those associated with leisure and stress-alleviating practices, with decreased risk for mental disorders. Finally, we hypothesized that a greater understanding of the COVID-19 (including hygiene and physical distance behaviors and agreement with the quarantine) would protect from mental illness, as this could decrease the fear of the pandemic (Brooks et al., Reference Brooks, Webster, Smith, Woodland, Wessely, Greenberg and Rubin2020) and enhance general cooperativeness (i.e. ‘collective effervescence’, as characterized by Durkheim) (Zumeta et al., Reference Zumeta, Castro-Abril, Méndez, Pizarro, Włodarczyk, Basabe and Pinto2020).
Methods
Study design
ELSA-Brasil is a prospective, longitudinal cohort of 15 105 participants from six universities in major Brazilian cities (São Paulo, Rio de Janeiro, Salvador, Belo Horizonte, Vitoria, and Porto Alegre). At its inception, it was the first, largest cohort in Latin America. Its aims were to identify the clinical and sociodemographic determinants of mortality and of the development of chronic diseases within a population of a low-/middle-income country. It initially aimed to recruit 15 000 out of 52 137 potential participants, stratified by sex, age, and occupational category. Recruitment goals were defined by sex (50% each), age (15% aged 35–44, 30% aged 45–54, 40% aged 55–64, and 15% aged 65–74 years) and occupational category (35% of support level, with incomplete elementary school; 35% with high school and 30% with higher education/teaching level). From 16 435 interested participants, 15 821 were pre-enrolled, and gave written consent, responding to an initial pre-interview. Only 716 (4.5%) of them did not complete the baseline examination, achieving a final sample of 15 105 participants. The recruitment goals were fully achieved in all centers (Schmidt et al., Reference Schmidt, Duncan, Mill, Lotufo, Chor, Barreto and Bensenor2014).
The cohort began in August 2008, when eligible participants were all active or retired employees of these universities, who were between 35 and 74 years old, and free of major neurocognitive disorders at enrollment (Aquino et al., Reference Aquino, Barreto, Bensenor, Carvalho, Chor, Duncan and Szklo2012; Schmidt et al., Reference Schmidt, Duncan, Mill, Lotufo, Chor, Barreto and Bensenor2014). Posterior waves did not recruit new participants. The first, second, and third waves occurred in 2008–2010, 2012–2014, and 2016–2018, respectively. During each wave, onsite assessments comprised of clinical interviews and examinations, collecting information on sociodemographic variables, clinical history, family history of diseases, lifestyle factors, and anthropometric measurements. Laboratory tests were also collected during the visits (Aquino et al., Reference Aquino, Barreto, Bensenor, Carvalho, Chor, Duncan and Szklo2012; Schmidt et al., Reference Schmidt, Duncan, Mill, Lotufo, Chor, Barreto and Bensenor2014).
In 2020, ‘COVID-19 wave’ assessments were carried out only by the São Paulo research center. Data collected during 2020 consisted of three online assessments (c1, c2, and c3 waves, respectively, performed between 18 May to 18 July; 20 July to 30 September; and 1 October to 22 December). The most severe lockdown measures in São Paulo started on 22 March 2020 and continued through 10 July 2020 (Quarentena, n.d.); therefore, c1 wave corresponds to the 8 to 16 quarantine weeks; c2 wave corresponds to an exponential increase of deaths and cases in Brazil, with some flexibility on quarantine measures adopted by the end of September and c3 wave corresponds to a moderate decrease in the rate of daily deaths and cases and greater quarantine relaxation measures in Brazil.
This study was approved by the Local Ethics Committee at the University Hospital, University of São Paulo and is reported according to the STROBE guidelines (von Elm et al., Reference von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke2007). All patients provided electronic informed consent for participation in the study.
Participants
Participants in the São Paulo center are active or retired public servants from the University of São Paulo, which remained physically closed from 20 March 2020 until the end of that year, with most activities being performed virtually, except for essential healthcare and research.
The study was advertised in the university newspapers and social media. All participants enrolled at the São Paulo research center who completed the third wave and could answer online surveys (i.e. internet availability and having a smartphone, tablet, or personal computer) were eligible and initially contacted via their personal or work emails using the RedCap platform (Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009). If they did not reply to three emails sent at weekly intervals, we additionally tried to contact them via three text messages (or via telephone calls if mobile phone numbers were not available) also sent at weekly intervals. Telephonic interviews were done if participants explicitly requested them due to difficulties in understanding or completing online questionnaires.
Variables
Outcome variables
Psychiatric diagnoses were assessed using the validated Brazilian version of the Clinical Interview Schedule-Revised, CIS-R (Lewis, Pelosi, Araya, & Dunn, Reference Lewis, Pelosi, Araya and Dunn1992; Nunes, Alves, Chor, Schimdt, & Duncan, Reference Nunes, Alves, Chor, Schimdt and Duncan2011), a structured interview for measurement and diagnosis of current non-psychotic psychiatric morbidity in the community. CIS-R has poor sensitivity for diagnosing mental disorders, which might slightly underestimate the rates of psychiatric disorders, although its specificity is high (Brugha et al., Reference Brugha, Bebbington, Jenkins, Meltzer, Taub, Janas and Vernon1999). Due to its length, it was applied only during the first COVID-19 wave assessment.
The CIS-R includes the assessment of 14 symptoms and 13 psychiatric disorders based on the International Classification of Disease, 10th edition (ICD-10). The CIS-R domains are somatic complaints, fatigue, concentration and forgetfulness, sleep disturbance, irritability, worry about physical health, depression, depression ideas, worry, anxiety, phobias, panic attacks, compulsions, and obsessions. Scores for each section range from 0 to 4 (except for the score for depressive ideas that range from 0 to 5); therefore, the total score ranges from 0 to 57. A symptom is present if the corresponding section score is ⩾2.
The relevant symptoms are grouped to form, with accessory questions and based on an algorithm following ICD-10 diagnostic criteria, the following diagnoses: mild depressive episode without (F32.00) and with somatic syndrome (F32.01); moderate depressive episode without (F32.10) and with somatic syndrome (F32.11); severe depressive episode (F32.2); agoraphobia without (F40.00) and with panic disorder (F40.01); social anxiety disorder (SAD, F40.1); specific (isolated) phobias (F40.2); panic disorder (PD, F41.0); generalized anxiety disorder (GAD, F41.1); and obsessive-compulsive disorder (OCD, F42.9). Finally, F32.xx diagnoses were collapsed in ‘depressive disorders’, and F40.xx and F41.xx diagnoses were collapsed in ‘anxiety disorders’.
Moreover, the total CIS-R score is obtained by adding the scores of all 14 (non-binarized) symptoms. Based on this score, a diagnosis of common mental disorder (CMD) (CIS-R > 11) is operationally defined (Lewis et al., Reference Lewis, Pelosi, Araya and Dunn1992). Finally, the CIS-R score of depressive symptoms was calculated by summing up symptom scores of depression, depression ideas, fatigue, concentration/forgetfulness, and sleep disturbance as used previously (Brunoni et al., Reference Brunoni, Salum, Hoffmann, Goulart, Barreto, Canhada and Benseñor2020; Khandaker, Zammit, Burgess, Lewis, & Jones, Reference Khandaker, Zammit, Burgess, Lewis and Jones2018).
Due to the quarantine measures, it was impossible to collect CIS-R data onsite, as done in previous waves. Therefore, we used an electronic, self-applied CIS-R format that was identical to the one used in clinical interviews. Importantly, the online version was self-applied, whereas the onsite version was read by trained personnel. Thus, uncontrolled differences in answering engagement could have occurred. However, previous studies have already validated and compared an electronic, self-applied CIS-R version with its standard format, showing that the electronic version presents valid and reliable performance (Lewis, Reference Lewis1994; Lewis et al., Reference Lewis, Pelosi, Glover, Wilkinson, Stansfeld, Williams and Shepherd1988). In fact, a validation study showed that the performance of both versions was similar (Head et al., Reference Head, Stansfeld, Ebmeier, Geddes, Allan, Lewis and Kivimäki2013). In that study, no differences between mean scores in 12 of the 14 symptom scores were observed. Moreover, both versions presented similar accuracy in diagnosing psychiatric disorders (Head et al., Reference Head, Stansfeld, Ebmeier, Geddes, Allan, Lewis and Kivimäki2013).
During the COVID-19 wave assessments (but not previously), we applied the Depression, Anxiety, and Stress Scales-21 (DASS-21) (Henry & Crawford, Reference Henry and Crawford2005), which is a self-reported set of three scales that measure symptoms of depression, anxiety, and stress. The scores of DASS-21 range from 0 to 63 and the symptoms’ subscores range from 0 to 21. Higher scores indicate greater severity.
Exposure variables
Sociodemographic data from the first wave of the ELSA-Brasil, such as birth year, sex, educational level (presence or absence of university degree), and self-reported ethnicity (White, Brown, Black, Indigenous, and Yellow) were used. Height (in cm) was collected onsite during the third wave; therefore, we used this information that we judged less prone to bias than self-reported height, even considering eventual height changes occurring between 2016–2018 and 2020. Current participant weight (in kg) was assessed in the c1 wave survey. Body mass index (BMI) was obtained dividing weight by squared height (kg/m2). Obesity was defined as BMI ⩾30.
Using additional information collected in the c1 wave survey, we codified another 24 exposure variables assessing participant home situation (which we labeled loneliness-related variables), comorbidities, distress caused by the pandemic, behaviors related to it, and factors related to being exposed to the virus (Table 1) (for more details, see online Supplementary material).
Table 1. Exposure variables
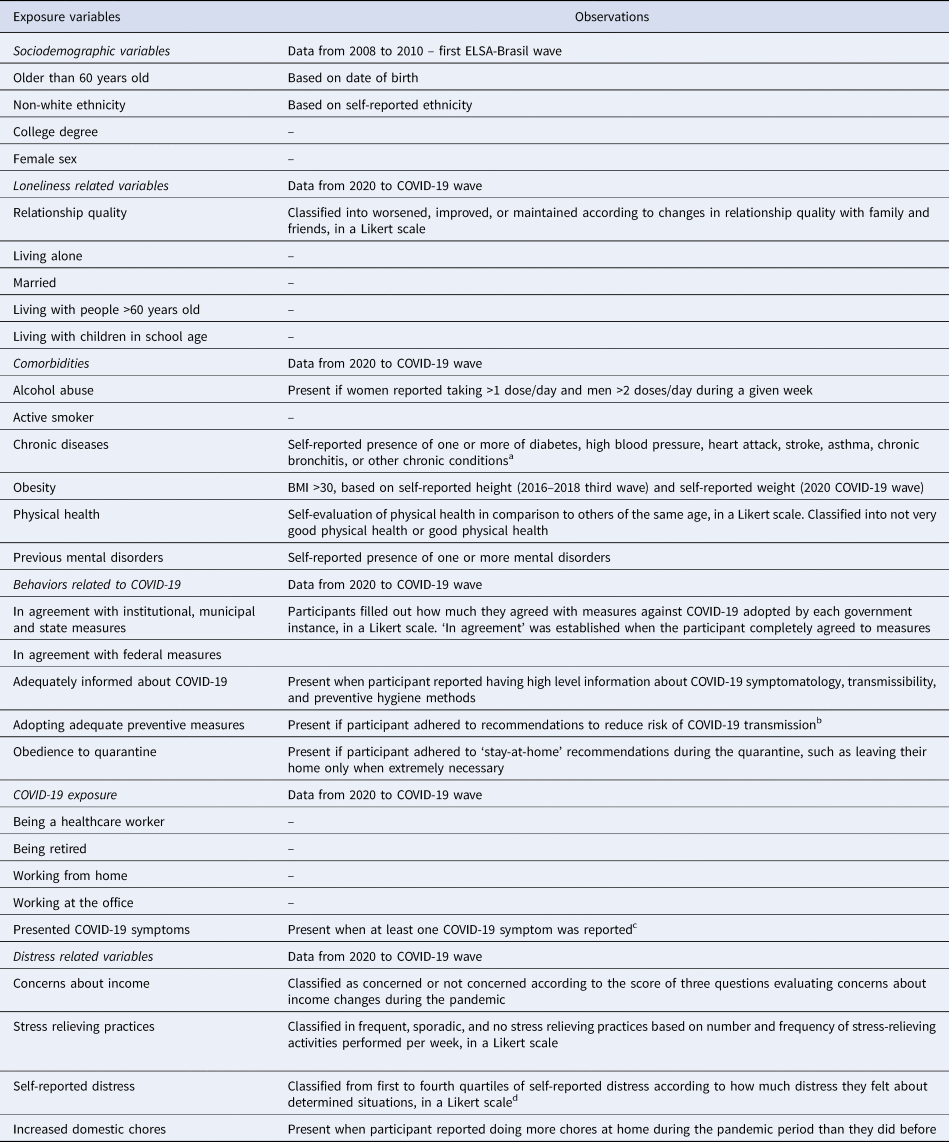
a If a chronic disorder was described, we assessed whether it could be considered as one.
b Recommendations were: washing hands frequently, removing shoes before entering their homes, wearing masks, covering mouth and nose when sneezing, refraining from shaking hands or kissing when greeting somebody, washing store bought packages before use, and using alcohol gel.
c COVID-19 symptoms were: fever, cough shortness of breath, sore throat, fatigue (physical), loss/decreased sense of smell, loss/decreased sense of taste.
d Situations were: staying home, avoiding close contact with other people or crowds, refraining from meeting friends, refraining from meeting other family members, having postponed or canceled important events, having postponed or canceled trips, and hearing news of the COVID-19 pandemic.
Analysis
At the inception of our study, there was no good-quality data available on the rate of psychiatric disorders during the COVID-19 pandemic. Therefore, we did not formally estimate a sample size based on a priori rates, but invited all eligible participants from the last onsite (third) wave. Statistical significance was set under an alpha threshold of 0.005. Accordingly, confidence intervals are reported in the 99.5% threshold (99.5% CI). Missing data were imputed as described in the online Supplementary material. For our first aim, we compared the rate of collapsed depressive disorders, anxiety disorders, OCD, and CMD between the pre-pandemic and pandemic assessments. We did not use information from wave 2, which did not assess the complete CIS-R. A Cochran's Q test for paired data was used to compare rates between waves, and post-hoc analyses were conducted applying pairwise McNemar tests. Also related to our first aim, we used the continuous scores of CIS-R to assess changes in depressive and total symptomatology during these assessments. For wave 2, we used only data from CIS-R depressive scores.
For our second aim, we used the DASS to assess symptoms of depression, anxiety, and stress, and overall symptoms during the three assessments performed in 2020. Symptom changes over assessments were evaluated using linear models, with time as the independent variable and DASS scores as the dependent variables.
For our third aim, we performed generalized linear models (binomial family, logit link) using the iteratively reweighted least squares method. One model was run for each exposure and outcome variables separately, and all models were adjusted by the covariates sex, age, educational level, and ethnicity. We also analyzed the influence of these covariates separately. The outcome variables were the collapsed depressive disorders, anxiety disorders, and CMD.
Results
Participants
Out of 4191 eligible participants from the 2016–2018 wave, data of 2117 participants (51.7%) could be included in our analyses. Reasons for non-inclusion were unwillingness to participate, impossibility of making contact, and deaths (Fig. 1). The included v. non-included sample had a significantly higher percentage of women, were younger, with a higher educational level and lower rates of psychiatric symptoms and diagnoses (online Supplementary Table S1). In the included sample, 450 (21.3%) presented CMD, 169 (8%) presented anxiety disorders, and 60 (2.8%) presented depressive disorders (Table 2).

Fig. 1. ELSA-Brasil São Paulo center flow chart. CIS-R, Clinical Interview Schedule-Revised; DASS, Depression, Anxiety, and Stress Scale. The full version of the CIS-R was applied in all waves, except for wave 2, when only questions regarding depressive symptoms were applied.
Table 2. Characteristics of the study sample

p Values are highlighted in bold when a significance of 0.005 was achieved in t tests or χ2 tests for continuous and categorical variables, respectively. Exposure variables are described in Table 1 and in the online Supplementary materials.
Aim 1: prevalence of diagnoses and symptomatology between pre-pandemic and pandemic assessments
We found no significant differences within the same sample in rates of CMD, and ICD-10-based diagnoses of depressive disorders and OCD between the first (2008–2010), third (2016–2018), and the COVID (May–July 2020) waves (Fig. 2). Anxiety rates decreased over time (first wave: 13.8%, third wave: 9.8%, COVID wave: 8.0%; Q = 50.58, p < 0.001), with significant differences between the first and the third waves (χ2 = 19.7, p adj < 0.001), and the first and the COVID waves (χ2 = 45.8, p adj < 0.001) (Fig. 2a).
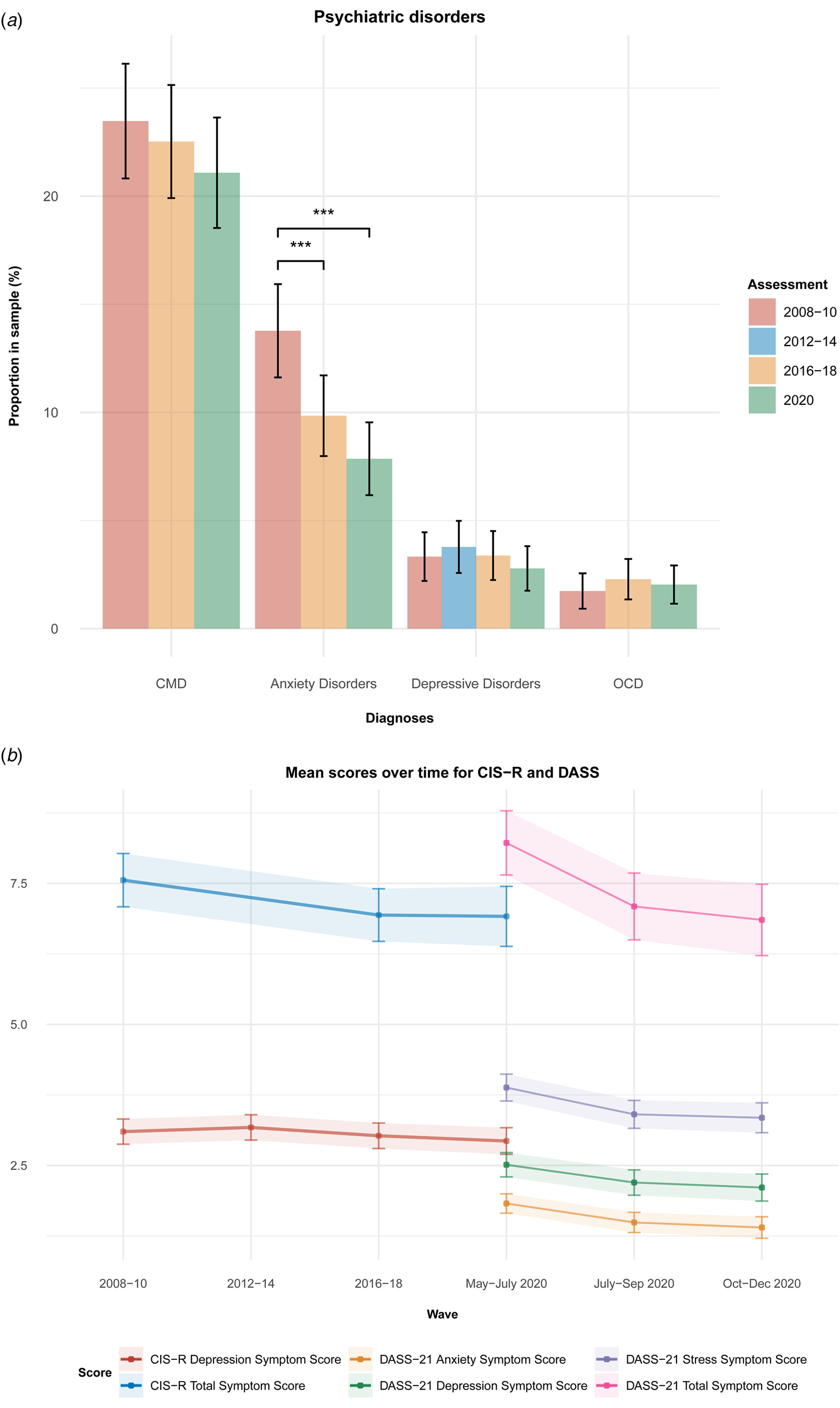
Fig. 2. Rates of psychiatric disorders and CIS-R total symptom scores at wave 1 (2008–2010), wave 2 (2012–2014), wave 3 (2016–2018) and first COVID wave (May–July 2020), and DASS depression, anxiety, stress, and overall total mental scores at first (May–July 2020), second (July–September 2020), and third (October–December 2020) COVID waves. (a) For psychiatric disorders, significant changes were only observed for anxiety disorders. (b) For symptoms, DASS scores decreased along COVID waves. Diagnoses were evaluated using the Clinical Interview Scheduled-Revised and are based on the International Classification of Diseases, 10th version (ICD-10). F32.xx diagnoses were collapsed in ‘depressive disorders’, and F40.xx and F41.xx diagnoses were collapsed in ‘anxiety disorders’. CMDs is a CIS-R-based classification that describes people with relevant mental symptoms (CIS-R score >11). CIS-R, Clinical Interview Schedule-Revised; DASS, Depression, Anxiety, and Stress Scale. Error bars represent 99.5% CIs.
CIS-R measurements of total symptom scores and depression scores did not significantly change over the assessments of the first (2008–2010), third (2016–2018), and COVID (May–July 2020) waves (Fig. 2b, online Supplementary Table S2).
Aim 2: changes in symptomatology during the pandemic in 2020
Significant decreases in DASS-21 scores were observed for total symptom (c3 v. c1: β = −1.22, 99.5% CI −1.58 to −0.86, p < 0.001), depression (c3 v. c1: β = −0.37, 99.5% CI −0.50 to −0.23, p < 0.001), anxiety (c3 v. c1: β = −0.37, 99.5% CI −0.48 to −0.26, p < 0.001), and stress scores (c3 v. c1: β = −0.48, 99.5% CI −0.64 to −0.33), p < 0.001) over time, although no significant changes between the second and third COVID wave assessments were observed (Fig. 2b, online Supplementary Table S2).
Aim 3: association between exposure variables and mental disorders
Regarding age, sex, ethnicity, and college degree; for CMD, being older and having completed college were associated with decreased risk, whereas non-white ethnic groups and women had increased risk (Fig. 3, online Supplementary Table S3). For depressive disorders, having a college degree was a protective factor (online Supplementary Fig. S1, Table S3). For anxiety disorders, older age, and having a college degree were protective factors (online Supplementary Fig. S2, Table S3).
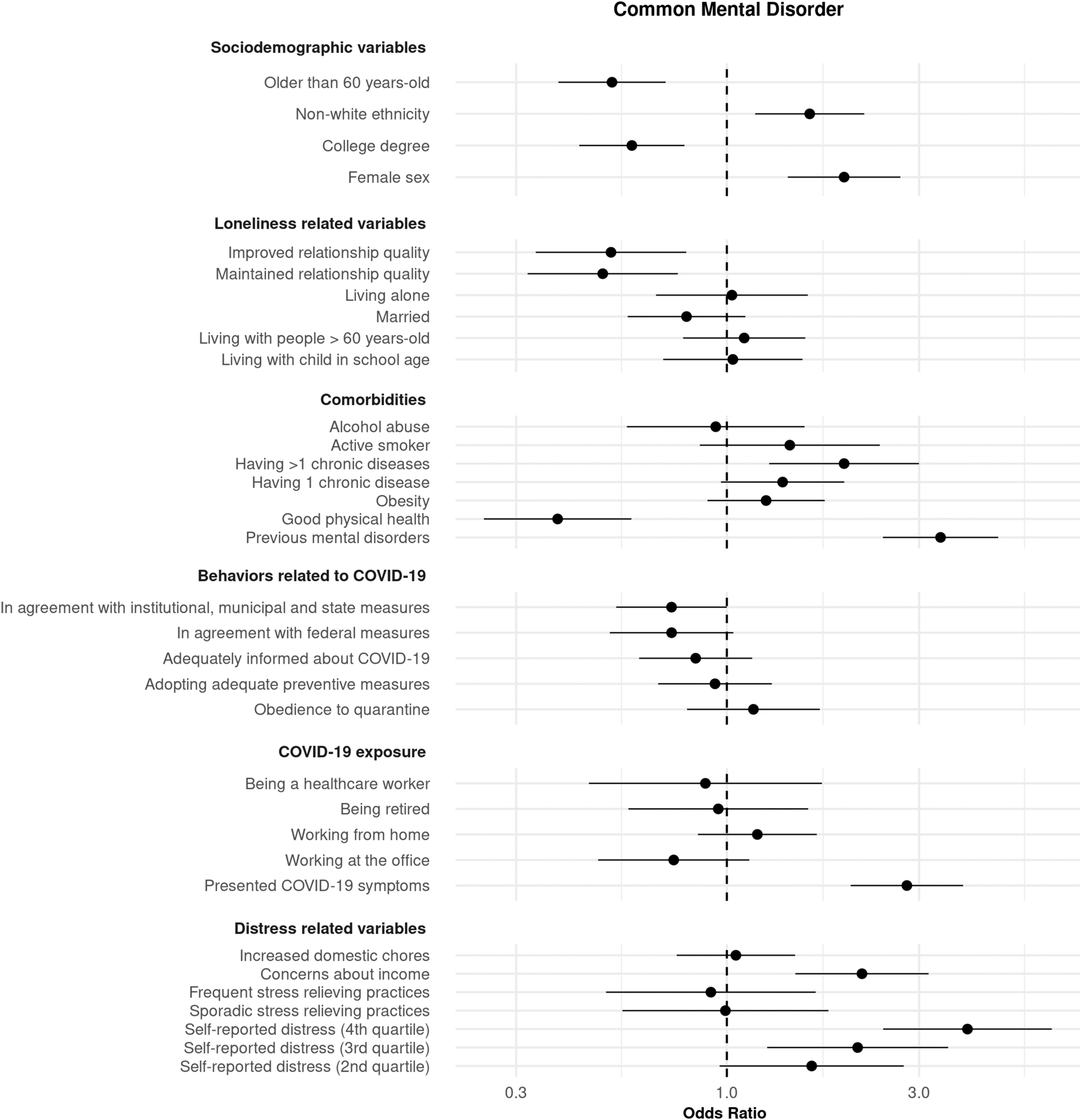
Fig. 3. Association of several exposure variables with CMDs. Association was measured using odds ratios (ORs) and 99.5% CIs. ORs > 1 and <1 indicate variables associated with increased and decreased risk, respectively. Models were adjusted by age, sex, ethnicity, and educational level.
Multivariable models adjusted by the abovementioned covariates showed, for CMD, a protective association for describing good physical health, describing maintained or improved quality of close relationships, and for alignment with institutional, municipal, and state measures. Conversely, associations that presented increased risk were having more than one chronic disease, being concerned about one's income during the pandemic, having had COVID-19 symptoms, describing elevated distress levels during the quarantine, and presenting mental disorders (Fig. 3, online Supplementary Table S3).
Multivariable analyses for depressive disorders showed that having had COVID-19 symptoms, and presenting mental disorders were associated with increased risk, whereas no factors were associated with decreased risk (online Supplementary Fig. S1, Table S3).
Multivariable analyses for anxiety disorders showed that having had COVID-19 symptoms, describing elevated distress levels during the quarantine, presenting mental disorders, being concerned about one's income during the pandemic, and having more than one chronic disease were associated with increased risk; whereas describing good physical health was associated with decreased risk (online Supplementary Fig. S2, Table S3).
Discussion
Our first aim was to compare the prevalence of mental disorders in 2117 participants in the ELSA-Brasil São Paulo study center between two pre-pandemic assessments (2008–2010 and 2016–2018) and the initial phases of the COVID-19 pandemic (May–July 2020). Contrary to our initial hypothesis, the rates of observed psychiatric disorders were not significantly different from previous assessments. Similarly, overall mental symptoms and depressive symptoms did not significantly change over time. In fact, a slight decrease in anxiety disorders was found. We believe that a lower rate in the COVID-19 assessment was partially observed due to a decrease in the rate of GAD, which has a time criterion duration of 6 months or more. As the assessment was performed in May–June and the pandemic started in March 2020, possibly some participants considered that anxiety symptoms only started after the pandemic, even if they were present before (recall bias). In fact, additional analyses changing the time criterion for 2 weeks or more (for all waves) revealed similar rates of anxiety disorders among all waves (data not shown).
As for our second aim, we performed three longitudinal assessments from May to December 2020 to evaluate changes in depression, anxiety, and stress scores. Also contrary to our hypothesis, these symptoms were either maintained or slightly decreased during the pandemic. Notwithstanding, our findings are in line with other studies. In a prospective study in the UK with more than 70 000 people examined during the lockdown period in the first semester of 2020, depressive and anxiety symptoms were moderately high at the beginning of lockdown measures but decreased rapidly during the next 20 weeks (Fancourt, Steptoe, & Bu, Reference Fancourt, Steptoe and Bu2020). A longitudinal study of three Dutch case-control cohorts, with well-characterized psychiatric disorders, employed data from approximately 1500 subjects and assessed depressive symptoms, anxiety, worry, and loneliness. People without psychiatric disorders showed a slight increase in symptoms during the pandemic, whereas those with the greatest previous mental health burden tended to exhibit a slight symptom decrease (Pan et al., Reference Pan, Kok, Eikelenboom, Horsfall, Jörg, Luteijn and Penninx2020). In a longitudinal assessment in Ireland with over 1000 participants (Hyland et al., Reference Hyland, Shevlin, Murphy, McBride, Fox, Bondjers and Vallières2020), rates of GAD were actually higher in 2019 compared to 2020, and further decreased during the pandemic. In contrast, other studies showed an increase in mental health symptomatology. In a US study comparing matched (but different) adult samples before and during the pandemic, the prevalence of probable depression rose from 8.5% to 27.8% (Ettman et al., Reference Ettman, Abdalla, Cohen, Sampson, Vivier and Galea2020). In a UK study with 48 486 respondents, the prevalence of mental health symptoms rose from 24.3% in 2016–2018 to 37.8% in April 2020, further decreasing to 31.9% in June 2020. Interestingly, those with a pre-existing depressive disorder did not experience an increase in mental symptoms (Daly, Sutin, & Robinson, Reference Daly, Sutin and Robinson2020).
Regarding the third aim, we confirmed, in agreement with our hypothesis, that female sex, non-white ethnicity and lower educational level were risk factors for CMD. These characteristics reflect socioeconomic disadvantages and have been observed as risk factors for incident and persistent depression in a previous ELSA-Brasil study (Brunoni et al., Reference Brunoni, Santos, Passos, Goulart, Koyanagi, Carvalho and Benseñor2019; Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020). People with lower educational levels also experienced more psychiatric symptoms (Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020), possibly due to accumulating workload and the impossibility of stopping working and/or working from home, generating distress. In a UK study, non-white ethnicities (Proto & Quintana-Domeque, Reference Proto and Quintana-Domeque2021) also experienced higher mental distress, in line with our findings. Interestingly, meta-analyses did not find an association between these factors and psychiatric symptoms (Prati & Mancini, Reference Prati and Mancini2021; Wu et al., Reference Wu, Jia, Shi, Niu, Yin, Xie and Wang2021), although meta-analyses are usually underpowered for such analyses. Although we expected that increased age – due to stress of presenting a severe form of COVID-19 – would be associated with psychiatric disorders, what was found was that people younger than 60 years old presented increased risk. Interestingly, this was also observed in a systematic review (Xiong et al., Reference Xiong, Lipsitz, Nasri, Lui, Gill, Phan and McIntyre2020) and could be explained by factors such as the impossibility of staying at home due to employment, lower financial support, and more domestic activities (e.g. taking care of children).
We also analyzed several other variables corrected for age, sex, ethnicity, and educational level. Psychiatric comorbidity was a risk factor for presenting CMD, reflecting the greatest mental burden associated with these patients, as demonstrated previously (Pan et al., Reference Pan, Kok, Eikelenboom, Horsfall, Jörg, Luteijn and Penninx2020), and emphasizing the need of maintaining psychiatric care during the pandemic. Regarding clinical comorbidities, exposure variables associated with increased rates of CMD include having had COVID-19 symptoms (tests were not widely available and there were stay-at-home instructions for mild cases when data were collected, so no confirmatory assessment was done), and presenting one or more chronic diseases, while self-reported good physical health was associated with decreased risk. This suggests that participants with greater susceptibility of presenting a severe case of COVID-19 were those more likely to develop psychiatric disorders. Conversely, obesity, being a smoker, and alcohol abuse were not associated with significant increased risk, which could be explained by inadequate perception or awareness of these variables as risk factors for severe COVID-19.
High or very high self-reported distress levels (evaluated by questions such as the distress associated with staying at home, avoiding contact with people, refraining from meeting friends and relatives, and others) and concerns about income were associated with increased odds of presenting mental disorders. However, stress alleviating practices were not associated with decreased risk, or increased domestic chores with increased risk. This possibly reveals that activities at home during the quarantine are less important for modifying mental health than initially hypothesized by researchers (Pfefferbaum & North, Reference Pfefferbaum and North2020). Notwithstanding, maintaining or improving relationship quality (e.g. using social apps) were associated with decreased risk. In fact, feelings of loneliness might have been attenuated via social media and electronic apps of mental health support; also, activities that could be done at home were not stopped (Williams et al., Reference Williams, Townson, Kapur, Ferreira, Nunn, Galante and Usher-Smith2021).
Interestingly, agreement with federal measures (which almost reached statistical significance) or institutional/municipal/state measures were independently associated with lower odds of having mental disorders. This is surprising as the Brazilian president adopted a radical anti-quarantine attitude, promoting mass rallies and systematically undermining the severity of the pandemic (The Lancet, 2020a), whereas the institutional/municipal/state instances adopted a pro-quarantine/pro-science perspective. Possibly, political identity and ideology are protective factors since they are cognitive shortcuts to support shared beliefs and similar choices (Pereira, Medeiros, & Bertholini, Reference Pereira, Medeiros and Bertholini2020), decreasing the mental burden in choosing between difficult options.
Finally, healthcare workers presented no increased odds for psychiatric disorders. Importantly, healthcare professionals from our sample work at the University Hospital, which only treated cases of moderate severity, whereas severe cases of COVID-19 were transferred to a tertiary university hospital. This might have reduced the stress overload of our sample of healthcare workers who worked under relatively less stressful conditions. Also, those with higher risk of COVID-19 morbimortality were kept away from work. Interestingly, a recent systematic review showed that their rates of depression and anxiety during the pandemic are neither necessarily higher than the general population, nor increased compared to pre-pandemic levels (Liu et al., Reference Liu, Luo, Haase, Guo, Wang, Liu and Yang2020).
Limitations
First, the mean age of our sample was around 60 years old; therefore, our results might not be applicable to younger populations. Second, approximately only half of the eligible sample answered our survey. This is in line with cohorts in the UK and the Netherlands that presented response rates in online assessments during the pandemic of 25–55% (Evandrou, Falkingham, Qin, & Vlachantoni, Reference Evandrou, Falkingham, Qin and Vlachantoni2021; Pan et al., Reference Pan, Kok, Eikelenboom, Horsfall, Jörg, Luteijn and Penninx2020; Pierce et al., Reference Pierce, Hope, Ford, Hatch, Hotopf, John and Abel2020). These modest rates probably occurred due to the need of rapid organization for collecting timely data during the pandemic. In fact, initial response survey rates are typically around 30% and increase only after many contacts, which usually take several months (Fincham, Reference Fincham2008). As we aimed to capture the mental health of the sample in a short period of time, we only extended our recruitment for 2 months. Third, although the differences between responders and non-responders were mostly small, a higher educational level was observed in responders, which probably reflects the spectrum of digital literacy within the sample. Notwithstanding, inherent issues of our sample are its relatively old age and low digital literacy.
Generalizability
We used a well-defined cohort, which decreased the risk of selection bias, enhancing the external validity and generalizability of the findings, in contrast to snowball sampling. However, our sample is occupational and not population-based, being composed of public servants of the University of São Paulo. Their income, which is on average higher than the national income, was essentially unaffected during the pandemic. Thus, the rates of psychiatric disorders and symptoms should not be considered as nationally representative, but rather interpreted in the context of longitudinal changes within the same sample and associated risk factors. Nonetheless, even representing a fraction of the Brazilian population, our findings are interesting for similar samples from other developing countries that continually struggle with large socioeconomic inequalities, with vulnerable safety nets, and which have some of the highest COVID-19 excess mortality rates (The Lancet, 2020b), and for mega-cohort analyses exploring whether the observed exposure variables are of worldwide importance or country-dependent, which is important to develop comprehensive early intervention strategies in different contexts.
Conclusion
During a strict lockdown period in São Paulo in May–July 2020, no major changes in psychiatric disorders and symptoms have been detected compared to earlier assessments in 2008–2010 and 2016–2018. Moreover, symptoms of depression, anxiety, and stress decreased along three assessments performed from May to December in 2020. Risk factors representing socioeconomic disadvantages and predictors associated with distress and loneliness were associated with increased odds of psychiatric disorders. As further quarantine periods may extend into the future, our findings are important to identify subgroups at elevated risk. Finally, follow-up surveys are necessary to identify trajectories of these disorders during the pandemic and post-pandemic phases. (Also check the online Supplementary material for further information.)
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721001719
Acknowledgements
We thank the ELSA-Brasil staff for administrative support.
Financial support
This study was supported by a São Paulo Research State Foundation (FAPESP) grant (20/05441-9). ARB receives scholarships and support from FAPESP, the Brazilian National Council of Scientific Development (CNPq-1B), University of São Paulo Medical School (FMUSP), the UK Academy of Medical Sciences (Newton Advanced Fellowship), and the International Health Cohort Consortium (IHCC).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







