Conjugated linoleic acid (CLA) is a mixture of linoleic acid isomers with conjugated double bonds. This fatty acid is produced naturally in the rumen of ruminant animals by biohydrogenation of linoleic acid or syntheticallyReference Pariza, Park and Cook1. CLA was first identified when extracts from fried beef were found to be anticarcinogenicReference Pariza, Ashoor, Chu and Lund2. This effect was confirmed in animal and in vitro models of carcinogenesisReference Zu and Schut3–Reference Wong, Chef, Wong, Hosick, Boylston and Schultz8. Further studies showed other beneficial effects of this CLA, including protection against arteriosclerosis, immune stimulation and modulation of body compositionReference Lee, Kritchevsky and Pariza9–Reference Park, Allbright, Liu, Storkson, Cooj and Pariza12.
The main isomer of CLA in natural foods is cis-9, trans-11, but trans-10, cis-12 is the isomer that affects energy metabolism and body fat deposition and composition in miceReference West, Delany, Camet, Blohm, Truett and Scimeca11, Reference Park, Allbright, Liu, Storkson, Cooj and Pariza12. The main dietary source of CLA for man is ruminant meats, such as beef and lamb, and dairy products, such as milk and cheese. The mean estimated daily CLA intake is 0·3–2·6 g/dReference German, Dilard and Ward13, Reference Yu, Adams and Watkins14, but in recent years, dietary changes have resulted in a decrease of CLA consumption.
It has been proposed that supplementing food with CLA could benefit healthReference Nagao and Yanagita15. The effects of CLA on body weight and body composition in man are documented, although some clinical studies in this field have been short term or have included a small sample of subjectsReference Gaullier, Berven, Blankson and Gudmundsen16. Moreover, the type of CLA isomer and the dose used varied between studies which may account for the discordant results. While some studies using a mixture of the bioactive isomers cis-9, trans-11 and trans-10, cis-12, reported reductions in body fat mass (BFM)Reference Blankson, Stakkestad, Fagertum, Thom, Wadstein and Gudmunsen17–Reference Riserús, Arner, Brismar and Vessby20 or an increase in lean body massReference Blankson, Stakkestad, Fagertum, Thom, Wadstein and Gudmunsen17, Reference Smedman and Vessby18, Reference Berven, Bye, Hals and Karlsen21, other short-term studies showed no effect on body compositionReference Zambell, Keim, Van Loan, Gale, Benito, Kelley and Nelson22–Reference Malpuech-Brugere, Verboeket-van de Venne, Mensink, Arnal, Morio, Brandolini, Saebo, Lassel, Chardigny, Sebedio and Beaufrere25.
Here we examined the effect of a 50 % mixture of the two active CLA isomers (cis-9, trans-11 and trans-10, cis-12; Tonalin®, Naturlinea; Central Lechera Asturiana, Spain) added to a skimmed milk, on BFM, lean body mass, BMI and biochemical parameters related to the metabolic syndrome. Although no serious toxic effects of CLA have been reported, we also evaluated alterations in the hepatic and renal function in addition to changes in haematological parameters.
Methods
We performed a randomised, double-blind, placebo-controlled dietary intervention trial. Participants in the study were men and women (n 60), aged 35–65 years, with a BMI of 25–35 (kg/m2) and with a waist diameter >102 cm in men and >88 cm in women. Moreover, to be included in the study, subjects had to fulfil two additional National Cholesterol Education Program criteria for the metabolic syndrome, namely (1) systolic pressure >130 mmHg or diastolic blood pressure >85 mmHg; (2) fasting plasma glucose >110 mg/dl; (3) HDL-cholesterol < 50 mg/dl in women and < 40 mg/dl in men; (4) TAG>150 mg/dl. Otherwise participants were apparently healthy. Finally, participants had to show a stable weight defined as a body weight variation of < 5 % in the 3 months previous to the study. Exclusion criteria included: alcoholism, active thyroid disease, diabetes mellitus treated with insulin or drugs, renal or liver dysfunction, and malignant tumours or other serious diseases. Moreover, subjects on anti-obesity drugs, with dietary variations of over 10 % in kJ/d during the study, subjects taking adrenergic agonists (α, β or both), pregnant or lactating women and subjects participating in another dietetic study were also excluded. The study was approved by the Ethical Research Board of the Hospital Clinic. Patients gave their signed informed consent. All subjects were recruited either from a primary care centre or an outpatient clinic dealing with cardiovascular risk. Subjects were randomised to receive 3 g CLA (using a mixture of the bioactive isomers cis-9, trans-11 and trans-10, cis-12; Tonalin®; n 30) in 500 ml skimmed milk (0·3 % total fat mass; Central Lechera Asturiana) or placebo (500 ml of the same skimmed milk; n 30) every day for 12 weeks. Packages of milk were delivered monthly by a home delivery service. The study protocol is summarised in Fig. 1. In brief, demographic data were recorded when subjects began the study (at week − 6). Weight, waist circumference, BMI, blood pressure, dietary control and adverse effects were recorded on each monthly visit (week − 6, week 0, week 4, week 8, week 12). Body composition was analysed by dual-energy X-ray absorptiometry (DEXA; with a Lunar Prodigy and software version 5.6). BFM (trunk and total) and lean body mass (trunk and total) were assessed at each time-point.

Fig. 1 Study design.
Blood samples were obtained from fasting subjects in week − 6 to check whether they met the inclusion criteria and again at week 0 and week 12. All samples were analysed in the same laboratory. The following analytic variables were studied: metabolic syndrome parameters (HDL-cholesterol, TAG, fasting plasma glucose and fasting immunoreactive insulin) and security parameters (haematological profile, renal and hepatic function). Insulin sensitivity was estimated from the HOMA-IR index [HOMA-IR = fasting glucose (mmol/l) × fasting immunoreactive insulin (μU/ml)/22·5].
Compliance and changes in the current diet were assessed through 3 d diet records on each visit. Each subject also completed a FFQ at weeks − 6, 0 and 12. A dietitian provided general counselling at the beginning of the study with detailed instruction on how to complete the FFQ, but no specific limits were set regarding energy intake or dietary habits. A software program, Dietsource® (Novartis, Switzerland) was used to calculate energy intake and macro- and micro-nutrient distribution. The dietitian also gave recommendations about exercise which was monitored throughout the study using the International Physical Activity QuestionnaireReference Craig, Marshall, Sjostrom, Bauman, Booth, Ainsworth, Pratt, Ekelund, Yngve, Sallis and Oja26. Three exercise categories were established (low, medium and intensive). Since the main aim of the study was to test the effects of CLA on body composition, subjects were excluded from the study when their total daily energy intake varied more than 10 % or when the variation in energy intake had resulted in a weight change >5 % during the study.
Statistical analyses were performed using the SPSS software package version 11.0 (SPSS Inc., Chicago, IL, USA). Changes in anthropometric and analytical variables from baseline to week 12 in each study group were assessed by paired Student's t-test. An unpaired Student's t-test was used to compare the mean change in each study variable between the two treatment groups. ANOVA was used to test the overall effect of the CLA supplementation and the interaction between CLA and other parameters. At baseline, differences in qualitative variables (such as sex or tobacco consumption) were tested through χ2; Student's t-test was used to assess differences in continuous variables (age). P < 0·05 was considered statistically significant.
Results
In our trial, ten participants from the CLA group and seven from the control group were excluded from analysis because of protocol violations: because of loss of follow-up (one participant from each group), dietary energy intake variations ≥ 10 % during the study (seven participants from the CLA group and four participants from the placebo group), weight reduction ≥ 5 % (one participant from the CLA group and two participants from the control group), both dietary and weight variations (one participant from the CLA group) (Fig. 2). There were no differences in exclusion percentages in the two groups (χ2 NS). Primary DEXA and analysis were performed in the ‘per protocol’ population. Patients lost to follow-up and patients with major protocol violations (those with dietary or weight changes outside the pre-defined boundaries) were excluded from the study.

Fig. 2 Study participant data. CLA, conjugated linoleic acid.
At baseline, mean age (CLA 54·79 (se 7·55) years; placebo 52·92 (se 7·92) years), sex (CLA 75 % male; placebo 78 % male) or tobacco consumption (CLA 31·5 % smokers; placebo 41·6 % smokers) showed no significant differences between the two groups. Alcohol consumption and exercise categories were also similar in the two groups. Likewise, at this time-point, metabolic syndrome variables (Table 1) did not differ significantly between the two groups. In the overweight subgroup, total fat tissue was significantly higher in the CLA than in the placebo group at week 0 (Table 2).
Table 1 Clinical values of metabolic syndrome of subjects participating in the conjugated linoleic acid (CLA) and placebo groups, at week 0 (pre-value) and week 12 (post-value) and stratified by BMI at the beginning of the study

Mean values were significantly different from the pre-values when comparing paired data within groups: *P = 0·02; **P = 0·009.
Mean value was significantly different from that of the placebo group: †P = 0·04.
§ HOMA-IR = fasting glucose (mmol/l) × fasting immunoreactive insulin (μU/ml)/22·5.
Table 2 Results of dual-energy X-ray absorptiometry, at week 0 (pre-value) and week 12 (post-value) and stratified by BMI at the beginning of the study
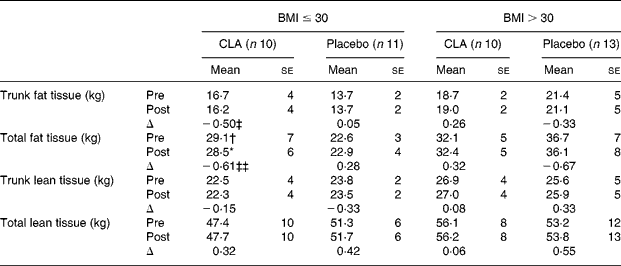
CLA, conjugated linoleic acid; Δ, change.
Mean value was significantly different from the pre-value when comparing paired data within groups: *P = 0·02.
Mean value was significantly different from those of the placebo group: †P = 0·03.
Δ Mean values were significantly different (CLA v. placebo): ‡P = 0·05; ‡‡P = 0·01.
Compliance with treatment was over 99 %. At week 12, daily energy intake, body weight and BMI had not altered from baseline values in either group.
When all subjects were considered, neither BFM (total fat tissue 30·6 to 30·5 kg in the CLA group; 30·3 to 30·0 kg in the placebo group) nor lean body mass (52·0 to 52·2 kg in the CLA group; 52·4 to 52·9 kg in the placebo group) differed significantly from baseline values. Gender did not affect these parameters. However, when subjects were stratified by BMI at week 0 (BMI>30 or ≤ 30 kg/m2), we observed a slight but significant decrease in total fat mass and a trend towards a decrease in trunk fat mass in the CLA subgroup when comparing paired data within cases (pre v. post values), while no changes in the placebo group were detected (Table 2). The differences were statistically significant when the change (Δ) in BFM parameters were compared between the two groups, CLA v. placebo. When ANOVA was used to test the overall effect of the CLA supplementation (F = 0·02; P = 0·8), BMI (F = 0·001; P = 0·9) and interaction between the two, the former interaction was statistically significant (F = 8·3; P = 0·006).
CLA supplementation was not associated with a significant change in any of the components of the metabolic syndrome compared with the control (Table 1). At week 12, there was no significant change in waist circumference, fasting plasma glucose, plasma TAG, total cholesterol, or systolic or diastolic blood pressure. LDL-cholesterol increased slightly in both CLA groups but these changes were not statistically significant. In contrast, in the subgroup of overweight patients, HDL increased in both groups. Insulin sensitivity at the end of the study period, as assessed from the HOMA-IR index, did not differ significantly from baseline levels. We did not observe a worsening in either hepatic or renal function markers, nor in haematological parameters in the CLA group (Table 3). We detected a significant reduction of alanine aminotransferase, a marker of liver disease in the CLA overall group (P = 0·01; results not shown). The statistical significance disappeared when the groups were stratified by BMI. Eight subjects in the control group (26·6 %) and one case (3·5 %) in the CLA group presented mild intestinal adverse effects (laxative effects and flatulence).
Table 3 Safety analysis, at week 0 (pre-value) and week 12 (post-value) and stratified by BMI at the beginning of the study

ALAT, alanine aminotransferase; AST, aspartate aminotransferase; CLA, conjugated linoleic acid.
Mean values were significantly different from those of the placebo group: †P = 0·03; ††P = 0·01.
Discussion
Milk supplementation with CLA (3 g/d) significantly reduces BFM after 12 weeks of treatment in overweight patients but not in grade I obesity subjects. Reductions affected both total and trunk values. We used DEXA technology to determine changes in body composition. This method has been validated by other studies and it can detect even small changes in body weight or composition.
The literature on the effects of CLA on body composition gives discordant results. Some of the factors that may account for disparity between studies are the type of CLA isomers used, the length of the treatment period and the type of patients enrolled. Table 4 shows some of the most relevant studies in this field. Most of the short-term assays (3 months) have been done with overweight patients. We believe that the lack of effect of CLA on BFM in obese subjects could be related to the short duration of the study. A 3- or 4-month study may not be long enough to detect all the changes caused by CLA. Only the study of Riserús et al. Reference Riserús, Berglund and Vessby23 showed significant changes in obese subjects (BMI ≥ 30) after 3 months of treatment. However, anthropometric measurements may not be reliable. The study of Gaullier et al. Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen27, Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen28, the only long-term one (12 months and 24-month follow-up), was performed on overweight subjects (average BMI between 27 and 28). These subjects showed reductions of 7·5 % in BFM from 6 to 12 months of treatment with CLA. If these changes in BFM are extrapolated to 3 months (Fig. 3), they would coincide with the 3 % decrease observed in the present study. In the present study most of the fat mass was lost from the trunk area, which is not surprising since individuals suffering from the metabolic syndrome usually present an accumulation of abdominal fat. The use of the DEXA technique allows not only the study of body composition but also the descriptive study of such modifications in the different areas of the body.
Table 4 Review comparing published results

BFM, body fat mass; c, cis; CLA, conjugated linoleic acid; DEXA, dual-energy X-ray absorptiometry; SAD, sagital abdominal diameter; t, trans.

Fig. 3 Fig. taken from Gaullier et al. Reference Gaullier, Halse, Hoye, Kristiansen, Fagertun, Vik and Gudmundsen27 and modified for the present study (·····). BFM, body fat mass.
It should be emphasized that this is the first study to be reported in which the mixture of two isomers was assessed in a large number of subjects and also the first in which the effects on body composition were measured using DEXA in both overweight and obese subjects.
It has been claimed that cis-9, trans-11, the main isomer found in the diet, modulates growthReference Pariza, Park and Cook29, and that trans-10, cis-12 has a beneficial effect on body composition and diabetes in miceReference Park, Storckson, Albright, Liu and Pariza30. Furthermore, trans-10, cis-12 also has been found to increase insulin resistance in a low percentage of human subjects with metabolic syndromeReference Riserús, Berglund and Vessby23.
The mechanisms by which CLA may affect body composition are unclear. CLA is believed to accumulate in tissues of animals and man, where it is readily metabolised. In vitro and in vivo studies report that the capacity of CLA to reduce adipose tissue can be explained by one or more of the following mechanisms: the induction of adipocyte apoptosisReference Evans, Geigerman, Cook, Curtis, Kuebler and McIntosh31; reduced accumulation of fatty acids in adipocytes because of an inhibition of lipoprotein lipase and an increase in carnitine palmitoyltransferase in serumReference Park and Pariza32; the binding to peroxisome proliferator-activated receptor present in fat tissue and modification of the signalling cascade to down-regulate leptin expressionReference Kallen and Lazar33; the prevention of the TAG accumulation in adipocytesReference Granlud, Juvet, Pedersen and Nebb34; and the modification of the metabolic rateReference West, Delany, Camet, Blohm, Truett and Scimeca11, Reference Szymczyk, Pisulewski, Szczurek and Hanczakowski35.
In the present study, energy intake slightly increased throughout the study in both experimental groups, although differences were not significant. This observation indicates that the effects of CLA on fat mass are diet-independent. Exercise and use of tobacco did not differ significantly between groups, and therefore neither factor can explain the changes in body composition observed in the CLA group.
Although CLA supplementation was associated with a change in body composition, it was not related to significant alterations of metabolic parameters. Previous studies on CLA report contradictory effects on blood lipids, which have recently been reviewedReference Salas-Salvado, Marquez-Sandoval and Bullo36. According to Tricon et al. Reference Tricon, Burdge and Kew37, trans-10, cis-12-CLA could increase both total and LDL-cholesterol concentrations more than the cis-9, trans-11-CLA isomer. However, none of the studies reviewed in this reportReference Salas-Salvado, Marquez-Sandoval and Bullo36 described significant effects attributable to CLA intake on the concentrations of total cholesterol or LDL-cholesterol. Smedman & VessbyReference Smedman and Vessby18 observed, as we did in the present study, increases in LDL-cholesterol levels in the group receiving CLA, although the changes observed were not significantly different from the control. In principle, non-significant changes should be considered as fluctuations within the normal physiological range. Moreover, in the present study they were not reproduced in the other BMI subgroup (>30). As to other possible modifications of the lipid profile, the literature contains references to HDL reductionReference Gaullier, Berven, Blankson and Gudmundsen16, Reference Nagao and Yanagita15, VLDL reductionReference Noone, Roche, Nugent and Gibney38 or any effect on cholesterolReference Berven, Bye, Hals and Karlsen21. In the present study, no significant variation in total cholesterol or TAG variations were observed. In a similar study, Basu et al. Reference Basu, Riserús, Turpeinen and Vessby39, Reference Basu, Smedman and Vessby40showed that men with metabolic syndrome increased F2-isoprostane excretion after supplementation with a CLA isomer mixture. This increased excretion returned to baseline levels 2 weeks after withdrawing CLA treatment. These findings indicate that CLA may induce lipid peroxidation; however, we did not test these possible effects in the present study.
We did not observe changes in fasting plasma glucose, blood pressure or insulin sensitivity estimates. Riserús et al. Reference Riserús, Arner, Brismar and Vessby20 showed an increased insulin resistance after 12 weeks of treatment with trans-10, cis-12-CLA isomer, in a male population with metabolic syndrome. Notably this effect was not observed when the mixture of isomers (Tonalin® mixture used in the present study) was usedReference Riserús, Arner, Brismar and Vessby20.
We detected a significant reduction of alanine aminotransferase in the overall CLA group but the statistical significance disappeared when the groups were stratified by BMI. Alanine aminotransferase can be considered a marker for fat liver degeneration, which can occur in obese subjects. Studies such as that by Tsuboyama-Kasaoka et al. Reference Tsuboyama-Kasaoka, Takahashi, Tanemura, Kim, Tange, Okuyama, Kasai, Ikemoto and Ezaki41 show that high doses of CLA induce liver enlargement and lipodystrophy in experimental animals. The dose of CLA like the ones used in the present study does not affect hepatic function, or may even favour the normalisation of certain enzymes.
We performed a ‘per protocol’ analysis on compliant patients with no major protocol violations, in order to quantify the effect of the intervention with a limited sample size. Patients were randomised when they entered the study, and so any slight variations between the subgroup studied at baseline are attributable to chance alone. For example, the CLA group with the lowest weight (BMI < 30) showed greater total fat mass in the DEXA compared with placebo group.
The degree of compliance was high in the present study, over 99 %. This high compliance rate demonstrates good tolerance of CLA milk (Naturlinea with Tonalin®). Only 3·5 % of the CLA group and 26·6 % of the control group showed slight adverse effects in gastrointestinal symptoms such as abdominal discomfort, laxative effects and flatulence. These adverse effects have been described by other authorsReference Blankson, Stakkestad, Fagertum, Thom, Wadstein and Gudmunsen17–Reference Riserús, Arner, Brismar and Vessby20. Moreover, the lack of alterations in haematological parameters and renal function further supports the security of milk supplemented with CLA.
In conclusion, 3 g CLA supplementation (Naturlinea with Tonalin®), over a 12-week period produced a significant reduction of fat mass in overweight subjects with a BMI ≤ 30. CLA supplementation was not associated with any adverse effects or biological changes. Further long-term studies are needed to evaluate more important reductions or to assess the effects in obese subjects. The present data indicate that CLA supplementation may be a useful approach to reduce fat mass, which is a prominent cardiovascular risk factor.
Acknowledgements
This study was supported by the Corporación Alimentaria Peñasanta, The Spanish Ministry of Health, Institute Carlos III (Ciber CB06/03, Fisiopatologia, obesidad y Nutrición) and DURSI GRC2005SGR00039. We especially wish to thank Roser Mestres, Blanca Valero, Isidora Torralba and Eva Suárez (Rosselló Primary Care Centre), María Mongay, Mònica Domenech and Cristina Sierra (Hypertension Unit, Hospital Clinic, Barcelona), and Ana Pérez, Berenice Cortés and Mercè Serra (Lipids Unit, Hospital Clinic, Barcelona) for facilitating the recruitment of patients.









