Introduction
Smartphone-based microscopes (also called mobile-phone microscopes) are in fashion for various purposes in several forms [Reference Perkel1]. For example, the LudusScope is designed as an accessible, low-cost education kit and can be constructed and expanded with ease. It is a playful educational tool capable of observing the structure and dynamic behavior of live microorganisms [Reference Kim2]. Another simple, cost-effective smartphone microscope platform has been developed for secondary and high school students to obtain research-level images of parts of insects, pollen grains, and sodium chloride crystals [Reference Wicks3]. One type of smartphone microscope is capable of identifying red and white blood cells in blood smears and soil-transmitted helminth eggs in stool samples [Reference Skandarajah4], and another smartphone microscope has been used to detect malaria in fixed and stained blood smears [Reference Pirnstill and Cote5,Reference Agbana6]. The CellScope and Newton Nm1 smartphone microscopes can be used to detect protozoa for the diagnosis of intestinal diseases [Reference Coulibaly7]. In the medical field, a tablet-based CellScope mobile microscope enables the remote diagnosis of oral cancer and may be used as a screening tool in pathology [Reference Skandarajah8]. Thus, smartphone microscopes seem to be solidifying their ground in the medical and public health fields.
We developed a novel type of mobile microscope extended from the Leeuwenhoek (L) simple microscope with a combination of a single (simple or compound) lens and a smartphone (Figure1). The new “L-eye mobile microscope” is compact and easy to handle, as it can be used while on a table and is free from blurring issues common with many types of mobile microscopes.
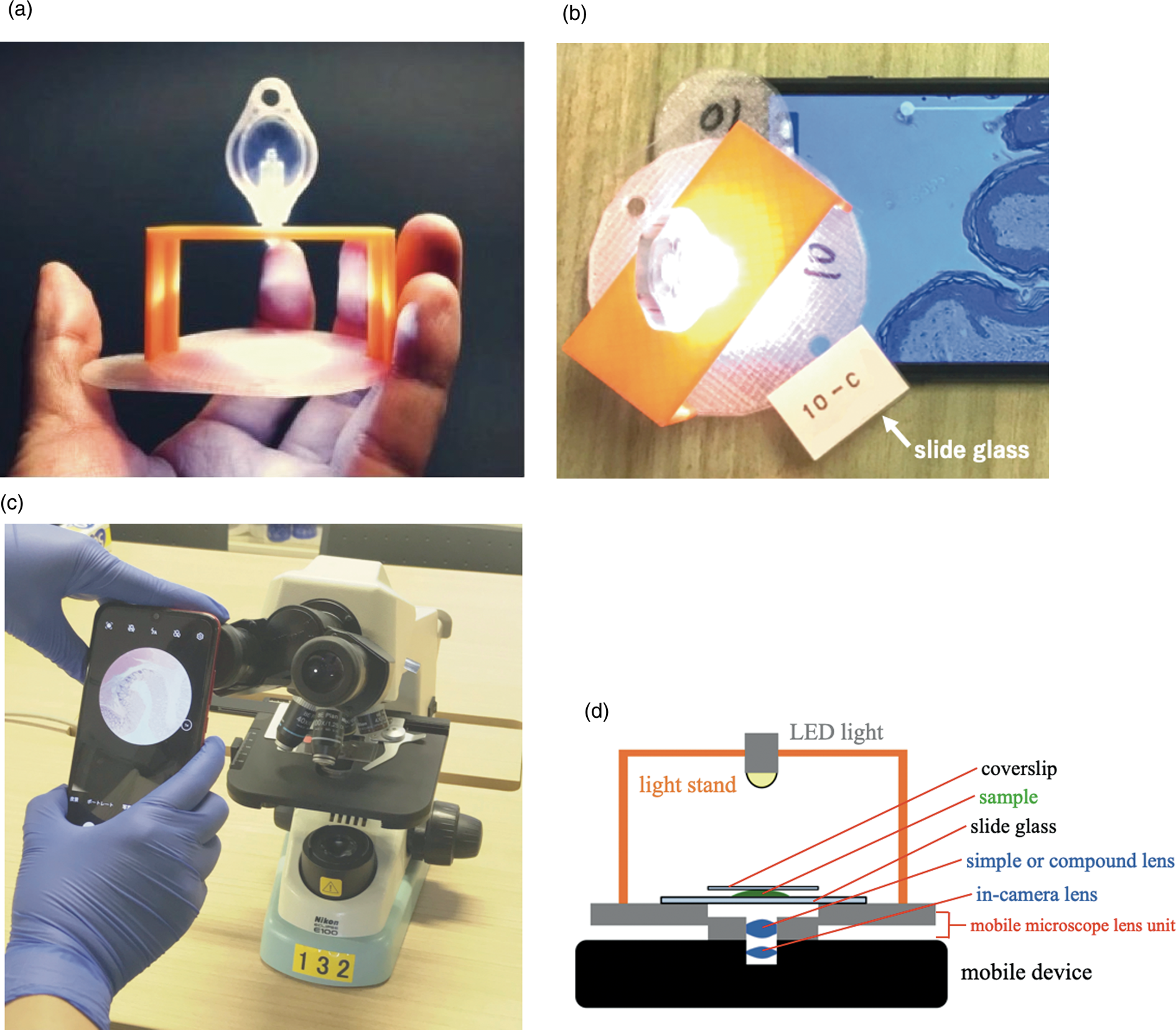
Figure 1: (a) An overview of the L-eye mobile microscope with an objective of 2.9 mm focal length. (b) A whole view of the L-eye microscope equipped with a smartphone (Huawei P10 Plus). (c) A conventional education-use microscope (ECLIPSE E100) that was mounted with a smartphone for image capture was the system used as a control. It was compared with the L-eye mobile microscope. (d) A schema of the side view of the L-eye microscope. The mobile microscope lens unit is a combination of a sample support made of a thin plastic disk with a female-screw in the center hole and a lens support holding a compound lens in the center with a male-screw outside. The screw mechanism enables focusing through the rotation of the thin-disk-attached light stand, which holds a LED.
The aim of this study was to investigate whether the new microscope can complement the use of conventional compound microscopes, which have traditionally been used for histology education. We made a comparative study with two kinds of microscope systems: the L-eye mobile microscope and a conventional microscope used for histology. Both systems were specifically mounted with the same smartphone for image capture. Since our concern was the performance of the core part of each microscope, the same camera and mobile device were employed for image capture.
Materials and Methods
Animals and tissue preparation.
For hematoxylin-eosin (H-E) staining, formalin-fixed skeletal muscle tissues of a postmortem male monkey (Macaca fuscata, five years old), which were kindly provided by Dr. Atsushi Nambu (National Institute for Physiological Sciences, NIPS), were washed with 0.1 M sodium phosphate / 0.15 M sodium chloride, pH 7.4, dehydrated in a graded ethanol and xylene series, and embedded in paraffin in a routine manner. Sections that were 4 µm thick were made on a sliding microtome and collected on 0.7 mm thick glass slides. The sections were rehydrated with xylene and ethanol in series and stained with Mayer's hematoxylin and eosin Y (Merck, Darmstadt, Germany). For Golgi-Cox staining, a whole male adult Wistar rat brain (Japan SLC, Hamamatsu, Japan) was placed in Golgi-Cox solution [Reference Koyama9] for two weeks and then sliced at 100 µm thickness on a microslicer (Dohan EM, Osaka, Japan), and developed with ammonia solution. All sections were dehydrated in a graded ethanol and xylene series and mounted with Entellan New (Merck, Damstadt, Germany) with 0.17 µm thick cover slips. The protocol for the monkey was carried out in compliance with the Animal Experimentation Regulations of the NIPS. The protocol for the rat was approved by the Institutional Animal Care and Use Committee (Protocol Number: #AP16082) and carried out according to the Fujita Health University Animal Experimentation Regulations.
Microscopy observations.
Two microscope systems were used for observation of the specimens. The first was a Leeuwenhoek-type mobile microscope (L-eye mobile microscope, Life is Small Company, Japan) (Figure 1), which includes a combination of an external lens made with a compound lens and a Huawei P10 Plus smartphone (Huawei P10 Plus, China). The other was a conventional microscope (ECLIPSE E100 microscope, Nikon, Japan) with the same Huawei P10 Plus smartphone directly attached to the eyepiece of the ECLIPSE E100. The mobile Huawei P10 Plus has three cameras: two back-facing cameras—one monochrome and one color—and a front-facing camera. While the color back-facing camera was mounted to the conventional microscope (ECLIPSE E100 microscope), the front-facing camera was used for the mobile microscope. The reason different cameras had to be used for the two microscope systems can be seen in Figures 1b and1c, where the front-facing camera is an integrated part of the mobile microscope and the back-facing camera is that of the smartphone-mounted conventional microscope.
Specifications of the lenses tested are listed together with the other performance data in Table 1. Magnification of the L-eye mobile microscope was adjusted by changing the focal length as shown in Table 1, and magnifications of ×180 and ×500 were used to test resolution. For the ECLIPSE E100 microscope, 10× and 40× objectives with a 10× intermediate lens were used for final magnifications of ×100 and ×400. For the resolution comparison for the two microscopes, the Edmund Optics (Barrington, NJ) test target was employed (Figure 2), and for the image quality investigation, tissue sections were imaged (Figures 3 and 4).

Figure 2: Images of a microscopy test target (Edmund) taken with (a) L-eye mobile microscope with an objective of 2.9 mm focal length (×180 magnification on the screen) has a resolution of 500 lp/mm or 2.0 µm; (b) L-eye mobile microscope with an objective of 0.75 mm focal length (×500 magnification on the screen) has a resolution of 700 lp/mm or 1.4 µm; (c) the ECLIPSE E100 with a 10× objective lens (×100 magnification) has a resolution of 600 lp/mm or 1.8 µm, and (d) the ECLIPSE E100 with a 40× objective lens (×400 magnification) has a resolution of 1100 lp/mm or 0.9 µm. Resolution with the L-eye mobile microscope is not quite as good as with the ECLIPSE E100 but sufficient for initial education in histology. Both microscopes were mounted with the same smartphone device (Huawei P10 Plus).

Figure 3: Images of 4 μm thick sections of monkey skeletal muscle with hematoxylin-eosin staining. (a), (b) An image taken with the L-eye mobile microscope, ×180(a) and ×500 (b). At the lower magnification muscle striations are not resolved, but with the ×500 magnification striations are visible. (c), (d) Images taken with the educational ECLIPSE E100 microscope with 10× (c) and 40× (d) objective lenses. As with the L-eye mobile system, striations are not visible at the lower resolution. All images were collected with the Huawei P10 Plus smartphone. Scale bars represent 50 μm.

Figure 4: Images of the same microscopic field of cells from a 100 µm section of rat cerebrum (Golgi-Cox staining). (a) An image taken with the ×180 mobile microscope showing excellent depth of focus on the thick section. (b) An image taken with the educational microscope ECLIPSE E100 with 10× objective lens. All images were collected with the Huawei P10 Plus smartphone. Scale bars represent 50 µm.
Table 1: Specifications of lenses used for comparison of the L-eye mobile and ECLIPSE E100 microscopes.

a: 0.61λ/NA (λ=589.6 nm (NaD line)).
b: Maker's specification for the objective lens.
c: Physical magnification at a Huawei photo-sensor coupled to a lens with a focal length of 3.0 mm. It gives a ×180 magnification on the screen of Huawei P10 Plus with ×6 pinch-out.
d: Physical magnification at a Huawei optical sensor coupled to a lens with a focal length of 3.0 mm. It gives ×500 magnification on the screen of Huawei P10 Plus with ×6 pinch-out.
e: A lens supplied from a Japanese company that is manufacturing small compound lenses for mobile cameras.
Results
Prior to the experiments, we tested the three cameras in the Huawei smartphone for image acquisition and found no obvious difference in image quality among them (data not shown).
The performance test with the Edmund test target is illustrated in Figure 2. The resolution of the ×180 (2.9 mm focal length) mobile microscope is approximately 500 lp/mm (2.0 µm resolution), and that of the ×500 mobile microscope (0.75 mm focal length) is approximately 700 lp/mm (1.4 µm resolution). Resolution of the ×100 ECLIPSE microscope (with a 10× objective lens) is about 600 lp/mm (1.8 µm resolution), and that of the ×400 ECLIPSE microscope (with a 40× objective lens) is about 1100 lp/mm (0.9 µm resolution). The resolution of the ×180 L-eye microscope is comparable to that of the ×500 L-eye microscope and slightly better than the ×100 ECLIPSE microscope, but the resolution of the ×500 L-eye microscope is worse than that of the ×400 ECLIPSE microscope.
Two types of sections customarily used for histology in medical education at Fujita Health University were embedded between a glass slide and a cover glass and examined with the L-eye (×180 and ×500) and educational (10× objective lens and 40× objective lens) microscopes. Figure 3 shows skeletal muscle from a monkey where characteristic long muscle fibers are seen with both the L-eye and educational microscopes. The striations of skeletal muscle fibers are visible with the ×500 L-eye microscope but not resolved with the 180× L-eye microscope (Figure 3a), which are compared with results obtained with the educational microscope (Figure 3b), where they are visible with the 40× objective lens but not resolved with the 10× lens. Figure 4 shows tissue from rat cerebrum where pyramidal cells with dendrites from the cell bodies are visible as a meshwork with both the L-eye mobile and ECLIPSE E100 microscopes. With the ×180 L-eye microscope all neurons in this thick section could be focused in the same image (Figure 4a). However, in the image from the ECLIPSE E100 microscope with a 10×objective lens, some neurons are focused, but others are out of focus (Figure 4b). The neurons imaged in this 100 µm section of brain are located at different distances from the objective.
Discussion
The resolution and image quality obtained by the L-eye mobile microscope are comparable to those obtained with the ECLIPSE E100. The resolution of the ×500 mobile microscope is sufficient to observe skeletal muscle fiber striations, which are not resolved with an educational microscope with a 10× objective (Figure3). The quality of the mobile microscope images for histological sections is good enough for initial histology education and understanding tissue structure. Furthermore, with the educational microscope with a 10× objective lens, all neurons are not necessarily focused in a single image (Figure 4b), but with the ×180 L-eye microscope they are focused in a single image (Figure 4b). The deeply focused image presentation of histological sections may make it easier for students to understand tissue structure.
Performance of the L-eye mobile microscope configured as a Leeuwenhoek-type system with the original small size was comparable to a conventional light microscope for histological observation. In contrast, most other smartphone microscopes are based on the combination of mobile devices and a traditional compound microscope, where the smartphone camera attaches to a long tube that holds intermediate and objective lenses and has the problem of blurring when taking a picture by hand. The blurring can be fixed with the use of a special bulky console, but only by sacrificing the original compactness. Compared to those smartphone microscopes, the L-eye mobile microscope may be distinguished by several characteristics: it is compact, light, portable, easy to operate and maintain, and, most importantly, free from the blurring issue as it is set statically on a table.
Many medical schools utilize one microscope for each student in histology and pathology classes. However, at paramedical educational sites such as nursing schools, a sufficient number of ordinary microscopes may not be available. Since the performance of the L-eye mobile microscope at a moderate magnification was found to be high enough to be practically employed, it could provide a handy and cost-effective substitute for standard microscopes used in nursing and paramedical settings. Furthermore, histology education with the L-eye mobile microscope can be extended to primary and secondary schools, to lectures for lay audiences, and to general use in underdeveloped countries where expensive microscopes are difficult to obtain.
Conclusions
The image quality, resolution, and colors of the pictures taken by the L-eye microscope, in spite of its simple construction, were comparable to those obtained by the conventional microscope used in medical education. The L-eye mobile microscope is convenient to use in medical, nursing, and paramedical educational settings as a secondary device, where the necessary number of ordinary microscopes are not available.
Acknowledgments
The authors are grateful to Prof. Atsushi Nambu of the National Institute for Physiological Science for providing tissue sections. We also would like to thank Mr. Toshiyuki Itoh for his continuous support for manufacturing mobile microscopes.