In 2023, even with the considerable distribution and access to coronavirus disease 2019 (COVID-19) vaccines, individuals are still at risk of infection. Reference Brown, Vostok and Johnson1,Reference Glatman-Freedman, Hershkovitz, Kaufman, Dichtiar, Keinan-Boker and Bromberg2 It has been proven that vaccination can reduce the incidence of serious outcomes, such as hospitalization and death. Reference Lopez Bernal, Andrews and Gower3,Reference Haas, Angulo and McLaughlin4
Healthcare workers have frequent exposure to COVID-19. 5,Reference Smeltzer, Copel and Bradley6 Even though the availability of personal protective equipment (PPE) is helpful in the prevention of infection, Reference Galanis, Vraka, Fragkou, Bilali and Kaitelidou7 access may be limited in resource-poor settings.
COVID-19 in this new phase of the pandemic can be partly explained by the relaxation of prevention measures that many countries had adopted in 2020 and 2021. Reference Lee, Kwon and Lee8–Reference Marra, Miraglia and Malheiros10 Some studies have concluded that a booster vaccine dose raises vaccine effectiveness considerably. Reference Arbel, Hammerman and Sergienko11,Reference Barda, Dagan and Cohen12 In Brazil, a significant share of the population received 2 doses of Oxford-AstraZeneca [ChAdOx1] or CoronaVac instead of mRNA vaccines because these vaccines were available first in many countries. In October 2021, our institution started administering mRNA vaccine boosters (Pfizer/BioNTech) for HCWs who had received either ChAdOx1 or CoronaVac as their primary series of 2 doses. Reference Marra, Miraglia and Malheiros10
In our previous study, we assessed the short-term (≤3 months) vaccine effectiveness of an mRNA vaccine booster following 2 doses of ChAdOx1 or CoronaVac against laboratory-confirmed COVID-19 among HCWs in Brazil. Reference Marra, Miraglia and Malheiros13 In this study, we have continued our evaluation of the effectiveness of an mRNA booster; we have extended the analysis over an 18-month period to evaluate the longer-term vaccine effectiveness in the SARS-CoV-2 delta and omicron variant eras.
Methods
Population and setting
We conducted a retrospective cohort study of all adult HCWs (aged ≥18 years) working at the Hospital Israelita Albert Einstein (HIAE) between January 1, 2021, and July 30, 2022. The HIAE is a Brazilian nonprofit healthcare, educational, and research organization headquartered in São Paulo. It manages diverse services from primary to tertiary care, in the public and private healthcare sectors, and it operates 40 healthcare units, mainly in the state of São Paulo. In 2020, the HIAE had 700,000 emergency department visits, 900,000 outpatient visits, and 70,000 hospital discharges. Since the beginning of the COVID-19 pandemic, HCWs with COVID-19 symptoms had access to free-of-charge SARS-CoV-2 RT-PCR testing conducted by the institution’s laboratory.
We included HCWs who completed at least 2 doses of either ChAdOx1 or CoronaVac vaccines, and we compared vaccine effectiveness in those who received an optional booster dose of mRNA vaccine to those who did not. HCWs were followed for 18 months (10 months following the booster dose). We excluded HCWs who no longer worked at HIAE, received just 1 dose of any COVID-19 vaccine, and received other combinations of COVID-19 vaccines (eg, Janssen vaccine + Pfizer/BioNTech vaccine), or received 4 doses of a COVID-19 vaccine (Supplementary Appendix 1).
Real-time polymerase chain reaction (RT-PCR) methodologies for SARS-CoV-2 detection
Diagnostic confirmation for COVID-19 was performed using RT-PCR on specimens obtained via nasopharyngeal swab, according to the protocol instituted at HIAE. The following RT-PCR kits were utilized: XGEN MASTER COVID-19 (Mobius, Pinhais, Paraná, Brazil), cobas SARS-CoV-2 Test (Roche Molecular Systems, Branchburg, NJ), Xpert Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA), and Abbott RealTime SARS-CoV-2 (Abbott Molecular, Des Plaines, IL).
Next-generation sequencing of the viral full-length genome
We extracted total nucleic acid from naso-oropharyngeal (NOP) swab samples with the QIAamp Viral RNA Mini kit (QIAGEN, Hilden, Germany). After purification and concentration, DNAse I treatment, and depletion of human ribosomal RNA, samples were submitted to random amplification. Reference Greninger, Naccache and Federman14 Preparation of sequencing libraries for the Illumina platform was carried out with DNA Prep (Illumina, San Diego, CA) using the random 2-step PCR amplification product as input. Libraries were quantified using the Qubit instrument (Thermo Fisher Scientific, Waltham, MA) and were loaded on the NextSeq 550 equipment (Illumina) for sequencing with MID 300 paired-end reads (Illumina).
Outcome measures and statistical analyses
Laboratory-confirmed COVID-19 was considered the primary outcome for calculating vaccine effectiveness. RT-PCR testing for the diagnosis of COVID-19 was performed only on symptomatic HCWs. Hospitalization related to COVID-19, length of stay, ICU admission, mechanical ventilation, and death were considered secondary outcomes. The vaccination status of all study participants and SARS-CoV-2 RT-PCR results of symptomatic HCWs were obtained from institutional electronic records. For those vaccinated, the initial follow-up date was 14 days after the second or the third vaccine dose. The last date was defined as the date COVID-19 was diagnosed, or up to July 30, 2022, for the censored cases without a positive diagnosis of COVID-19.
Qualitative variables were characterized using absolute and relative frequencies in general and by interest groups. For comparisons, we used the χ2 or Fisher exact tests. Quantitative variables were described by medians, interquartile range (IQR, first and third quartiles), and minimum and maximum values due to the asymmetry observed in the variables, Reference Altman15 and comparisons were performed using nonparametric Mann-Whitney tests. Vaccine effectiveness was defined as 1 − hazard rate (HR), Reference Nauta16 with HR determined by adjusting the survival analysis models with laboratory-confirmed COVID-19 as the outcome and vaccination and previous COVID-19 as the main explanatory variables. To assess the variation in the effectiveness of the booster dose over time (up to 250 days), we estimated the effectiveness rate by taking the log-risk ratio as a function of time (Figs. 1 and 2). These estimates considered sex, age, HCW job type (direct patient contact vs no direct patient contact), and comorbidities as covariates. HCWs with previous COVID-19 were excluded in the first model (Fig. 1), and they were included in the second model (Fig. 2). All analyses were performed using R software for statistical computing version 4.2.0 software, 17 DOVE software, Reference Lin, Zeng and Holloway18 and ggplot2. Reference Wickham19 All reported tests were 2-sided, and P < .01 was considered significant. The study was approved by the Hospital Israelita Albert Einstein Ethics Committee (CAAE 47110421.7.0000.0071), and the need for informed consent was waived.
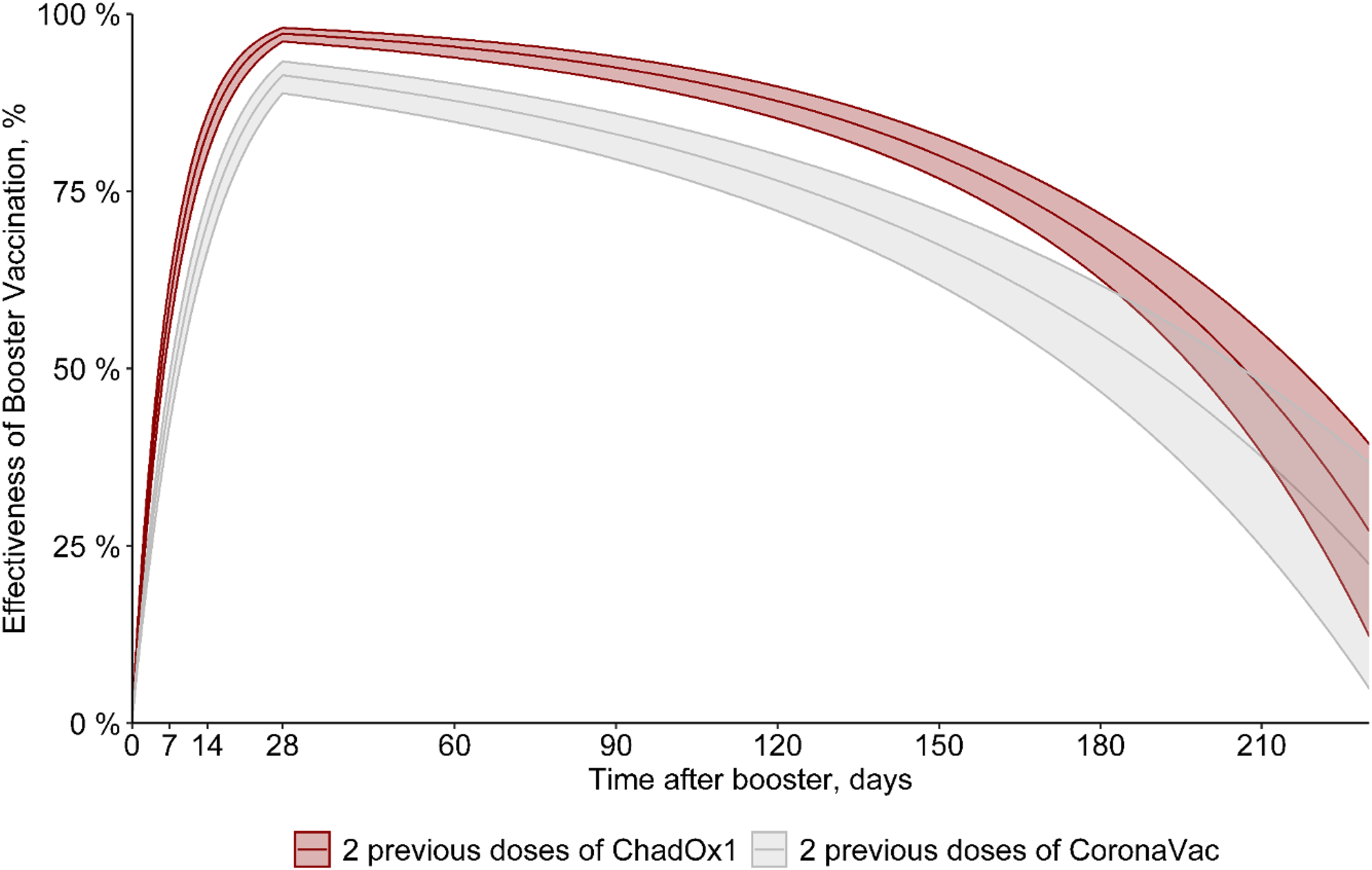
Figure 1. Effectiveness of 2 doses of Oxford-AstraZeneca (ChAdOx1) [red] or CoronaVac [gray] with a third (booster) dose with mRNA (Pfizer/BioNTech) vaccine, excluding those with previous COVID-19.

Figure 2. Effectiveness of 2 doses of Oxford-AstraZeneca (ChAdOx1) [green] or CoronaVac [purple] with a third (booster) dose with mRNA (Pfizer/BioNTech) vaccine, including those with previous COVID-19
Results
During the study period, 18,359 individuals were screened for eligibility and 14,532 HCWs met inclusion criteria (Supplementary Appendix 1). Most were female (70.7%), and the median age was 36 years. Of those included, 892 (6.1%) received 2 doses of CoronaVac vaccine, 6,285 (43.3%) received 2 doses of CoronaVac vaccine plus mRNA (Pfizer/BioNTech) booster, 1,111 (7.6%) received 2 doses of ChAdOx1 vaccine, and 6,244 (43.0%) received 2 doses of ChAdOx1 vaccine plus mRNA (Pfizer/BioNTech) booster.
Compared to the group that received 2 doses of CoronaVac vaccine, the group that received a Pfizer/BioNTech booster following CoronaVac vaccine was significantly older and had a greater proportion of HCWs with patient contact (Table 1). Compared to the group that received 2 doses of ChAdOx1 vaccine, the group that received a Pfizer/BioNTech booster following a ChAdOx1 primary series was significantly older, had a greater proportion of women, and had a smaller proportion of HCWs with patient contact.
Table 1. Baseline Characteristics of Study Participants

Note. ChAdOx1 vaccine, Oxford-AstraZeneca vaccine; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR: interquartile range; PCR, polymerase chain reaction.
a Comparisons for categorical variables performed with χ2 or Fisher exact test; for quantitative variables, the Mann-Whitney test was used.
b Information available for 13,975 participants (96.2%), 843 with 2 doses of CoronaVac vaccine, 998 with 2 doses of ChAdOx1 vaccine, 6,018 with 2 doses of CoronaVac vaccine + Pfizer/BioNTech vaccine and 6,116 with 2 doses of ChAdOx1 vaccine + Pfizer/BioNTech vaccine.
During the study period, 2,662 were diagnosed with COVID-19 prior to vaccination, and 3,785 had COVID-19 after vaccination. Overall, COVID-19 cases detected after vaccination occurred in 56.3% of HCWs who received 2 doses of the CoronaVac vaccine and 23.2% who received a booster (P < .001). COVID-19 cases detected after vaccination occurred in 37.1% of HCWs who received 2 doses of the ChAdOx1 vaccine and 22.7% who received a booster (P < .001).
Estimates of vaccine effectiveness of 2 doses of Oxford-AstraZeneca (ChAdOx1) or CoronaVac with a third (booster) dose with mRNA (Pfizer/BioNTech) vaccine are shown in Figure 1 and Table 2. The estimated vaccine effectiveness rates in the period beginning 15 days after receiving the mRNA booster dose were 73% for HCWs who received primary doses of the CoronaVac vaccine and 85% those for who received primary doses of the ChAdOx1 vaccine. The highest vaccine effectiveness rates (peak level) were observed 30 days after the booster dose (91% and 97%, respectively), with a progressive decrease in vaccine effectiveness after this period. At 180 days after a booster, the vaccine effectiveness rates were 55% and 67.5%, respectively (Fig. 1 and Table 2). In addition, 0.8% of HCWs who received 2 doses of CoronaVac had at least 1 hospitalization compared to 0.03% of HCWs who received a booster dose following CoronaVac vaccine (P < .001). On the other hand, no difference was observed in hospitalizations between those with 2 doses of the ChAdOx1 vaccine (2 cases, 0.18%) and those with a booster dose following ChAdOx1 vaccine (3 cases, 0.05%) (P = .16). There was no statistically significant difference between those with 2 doses (either ChAdOx1 or CoronaVac) and a booster dose in length of stay, ICU stays, or mechanical ventilation use (Table 1). Only 1 HCW vaccinated with 2 doses of the ChAdOx1 vaccine died during the study period, before the booster dose was released. This HCW was immunocompromised due to systemic lupus erythematosus treatment. When including those with COVID-19 prior to vaccinationin in the model, the vaccine effectiveness was quite similar to the first vaccine effectiveness model in which those with prior infection were excluded. The estimated vaccine effectiveness in those receiving the mRNA booster dose was 72% for HCWs who received primary doses of the CoronaVac vaccine and 84.5% those for who received primary doses of the ChAdOx1 vaccine. The highest vaccine effectiveness (peak level) was observed after 30 days (90.5% and 97%, respectively) with a progressive decrease in vaccine effectiveness after this period, and at 180 days, the vaccine effectiveness was 54.5% and 68%, respectively (Fig. 2 and Table 3).
Table 2. Estimated Effectiveness of a Third COVID-19 Vaccine Dose (Pfizer/BioNTech vaccine) in Reducing the Risk of Infection Over Time Among Healthcare Workers (HCWs) Without Previous COVID-19
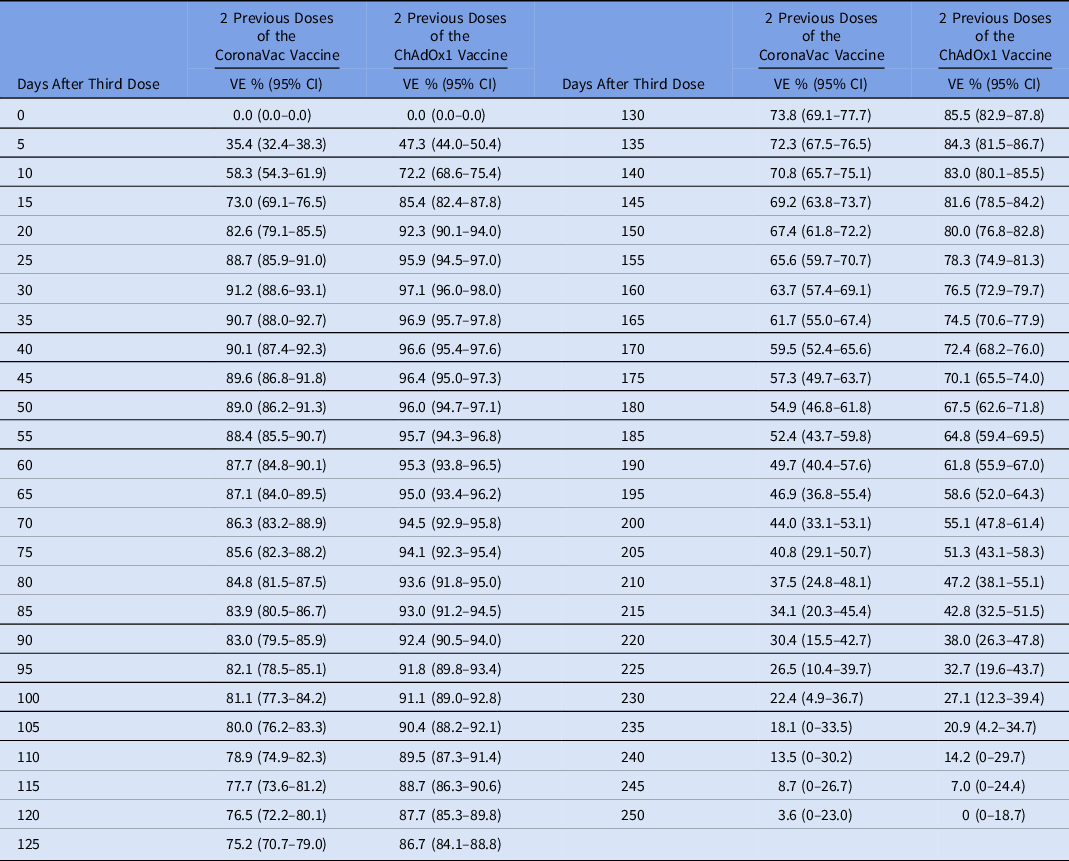
Note. These estimates considered sex, age, HCW job, and comorbidities as covariates. Cases with previous COVID-19 were excluded.
Table 3. Estimated Effectiveness of a Third COVID-19 Vaccine Dose (Pfizer/BioNTech vaccine) in Reducing the Risk of Infection Over Time Among Healthcare Workers (HCWs) Including Those With Previous COVID-19

Note. These estimates considered sex, age, HCW job, comorbidities and previous COVID-19 as covariates.
Whole-genome sequencing analysis
During the study period, 430 SARS-CoV-2 samples, the first collected from each HCW, were screened for mutations. One (0.2%) case was the SARS-CoV-2 alpha variant, 68 (15.8%) were P1 strain (Gamma SARS-CoV-2 variant), 213 (49.5%) were delta variant cases, and 147 (34.2%) were omicron variant cases. In November and December 2021, 90.5% of cases were the delta variant. Almost all cases (95.2%) were the omicron variant in January and February 2022, and 100% were the omicron variant from March to July 2022 (Table 4).
Table 4. Participants with SARS-CoV-2 Variants of Concern (n=430) Detected by Whole-Genome Sequencing

Note. VOC, variant of concern; WGS, whole-genome sequencing.
a Data presented as number (row percentage). The 430 samples screened for mutations were the first samples collected from each individual.
Discussion
In this retrospective study, longer-term vaccine effectiveness with heterologous COVID-19 vaccines (2 doses of either CoronaVac or ChAdOx1 vaccine followed by mRNA booster) was still reasonable for both vaccines, even after adjusting for important variables such as the time to event from the last dose (ie, exposure duration) and infection with the SARS-CoV-2 delta and omicron variants. However, protection after 3 doses of the COVID-19 vaccine waned overtime but provided good protection against infection, hospitalization and death, especially up to 6 months.
This result is endorsed by published evidence that the booster is capable of raising the protection already built by the primary doses. Rates of COVID-19 were significantly lower among people who received the booster than among those who have just been vaccinated with 2 doses. Reference Marra, Miraglia and Malheiros13,Reference Munro, Janani and Cornelius20,Reference Yigit, Ozkaya-Parlakay, Cosgun, Ince, Bulut and Senel21 A similar comparison of 3 versus 2 doses of SARS-CoV-2 vaccines found a high vaccine effectiveness of booster dose against symptomatic infection and demonstrated that the booster was safe. Reference Butt, Talisa, Yan, Shaikh, Omer and Mayr22 A case–control study corroborates that the booster adds significant protection. Reference Richterman, Behrman, Brennan, O’Donnell, Snider and Chaiyachati23 However, this study showed reduced vaccine effectiveness during the SARS-CoV-2 omicron variant period. Reference Richterman, Behrman, Brennan, O’Donnell, Snider and Chaiyachati23 Notably, these booster studies did not consider administration of heterologous COVID-19 vaccines in their analysis.
This waning immunogenicity of the vaccines over time was also reported in another study that evaluated the duration and effectiveness of immunity induced by 2 doses of mRNA vaccine and 2 doses of viral vector vaccine. Reference Hall, Foulkes and Insalata24 In the short term, the 2 doses promoted high protection against SARS-CoV-2 infection (vaccine effectiveness, 91%–97%). However, after 6 months, the protection decreased (vaccine effectiveness, 55%–67%) and in this period of waning of protection, the omicron variant was the predominant SARS-CoV-2 strain. This finding reinforces the importance of the booster doses after the primary doses of the vaccines to maintain protection, considering not only the waning immunogenicity but also the emergence of the new variants. Initial studies have shown that heterologous boostering may result in higher neutralizing-antibody responses than homologous boostering, particularly after primary doses with a viral vector vaccine. Reference Normark, Vikström and Gwon25,Reference Atmar, Lyke and Deming26 In a study of US veterans, heterologous mRNA boosting offered better protection against COVID-19 in individuals who were initially vaccinated with a viral vector vaccine. Reference Mayr, Talisa, Shaikh, Yende and Butt27 A Chilean study also demonstrated heterologous boosters showed higher vaccine effectiveness among individuals with a complete primary vaccination doses with CoronaVac for all outcomes, providing additional support for this vaccine strategy. Reference Jara, Undurraga and Zubizarreta28 Similarly, a recent study from Malaysa demonstrated that heterologous boosting using Pfizer/BioNTech vaccine after inactivated and viral vector primary vaccination is preferred. Reference Suah, Tng and Tok29 Thus, HCWs who received either Coronavac or ChAdOx1 primary vaccination should receive an mRNA booster, if available.
In our study, vaccine effectiveness among HCWs who had previous COVID-19 was similar to vaccine effectiveness among HCWs who did not have previous infection, and vaccine effectiveness among both groups declined over time. Therefore, the second booster should be recommended to HCWs who completed 3 doses regardless of whether they had COVID-19. In addition, vaccine effectiveness was nearly zero by the ninth month after the first booster, and a second booster is strongly encouraged for HCWs before the protection from prior vaccines completely fades away.
Our study had several limitations. First, this is an observational study, which is subject to multiple biases. Reference Harris, Lautenbach and Perencevich30 However, this is the most common study design in the infection prevention literature. Reference Harris, Lautenbach and Perencevich30 We did not perform a test-negative design case–control study because this study was retrospectively conducted using data from symptom-based testing. There is a possibility that HCWs had asymptomatic SARS-CoV-2 infection and did not undergo testing, leading to misclassification of the outcome. Reference Andrews, Stowe and Kirsebom31,Reference Cerqueira-Silva, Katikireddi and de Araujo Oliveira32 Second, we did not directly compare vaccine effectiveness between those with 2 doses of CoronaVac followed by mRNA vaccine and those with 2 doses of ChAdOx1 followed by mRNA vaccine. However, estimated vaccine effectiveness over time was comparable between the 2 groups with >50% of vaccine effectiveness up to 180 days after receiving the mRNA booster dose, thus a good time point to get a second booster (Figs. 1 and 2). Third, we were not able to compare homologous versus heterologous booster because most of our HCWs received heterologous COVID-19 vaccination. A previous study evaluating longer-term vaccine effectiveness detected that heterologous boosting was associated with greater protection than homologous boosting for those with mRNA vaccine primary dosing. Reference Lin, Gu and Xu33 However, a nationwide study from Brazil detected reduced longer-term vaccine effectiveness for homologous and heterologous (Pfizer/BioNTech COVID-19 vaccine) booster doses in preventing COVID-19 in adults who received primary doses of CoronaVac during the SARS-CoV-2 omicron variant period. Reference Ranzani, Hitchings and de Melo34 Fourth, we could not perform further analyses by immunocompromised status due to the limited number of cases. Fifth, neutralizing viral antigen-binding antibody levels were not available in our HCW cohort study. However, the US FDA does not recommend antibody testing for SARS-CoV-2 to determine immunity or protection from COVID-19, especially among those who are vaccinated. 35 Sixth, our study focused only on long-term vaccine effectiveness for the third dose against COVID-19 in HCWs; thus, we could not fully evaluate vaccine effectiveness for other outcomes such as COVID-19 hospitalization, COVID-19 reinfection, or COVID-19 death, because these outcomes were few in number. Other studies have demonstrated that the booster dose also has a significant protective effect against these severe outcomes. Reference Arbel, Hammerman and Sergienko11,Reference Bar-On, Goldberg and Mandel36 Lastly, we were not able to perform viral sequencing in all COVID-19 cases; it was limited to a small random sample of 430 HCWs, but the most prevalent variants detected in the study period were SARS-CoV-2 delta and omicron variants, representing ∼85% of cases.
In conclusion, viral vector and inactivated virus COVID-19 vaccines can significantly prevent infection, hospitalization, and death among HCWs when boosted with a third dose of Pfizer/BioNTech mRNA vaccine, even for a relatively long period (6 months). This heterologous vaccine strategy was also effective among HCWs even after emergence of a new SARS-CoV-2 variants (ie, omicron). The associated protection waned over 180 days, independent of having previous COVID-19, which suggests the necessity for a second booster. More studies are needed to evaluate vaccine effectiveness for other heterologous prime-booster COVID-19 vaccines (bivalent COVID-19 vaccines), COVID-19 breakthrough infection, and analysis of genomic surveillance to better understand vaccine effectiveness against newer SARS-CoV-2 variants, such as omicron BA.5 and XBB.1.5.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2023.173
Acknowledgments
We thank all the participants for contributing to this study.
Financial support
The sequencing reactions carried out to characterize the circulating SARS-CoV-2 described in this study were supported by grant 402669/2020-7 from Chamada MCTIC/CNPq/FNDCT/MS/SCTIE/Deceit 07/2020, Brazil.
Competing interests
All authors report no conflict of interest relevant to this article.








