Introduction
Chronic kidney disease (CKD) is a persistent decline of the kidney function and/or urinary abnormalities such as haematuria and proteinuria for over 3 months and affects 10–15% of the world population (Levey et al., Reference Levey, Eckardt, Dorman, Christiansen, Hoorn, Ingelfinger and Winkelmayer2020). The most severe stage is called end-stage kidney failure (ESKF), when patients will require haemodialysis (HD), peritoneal dialysis (PD) or kidney transplant (Bikbov et al., Reference Bikbov, Purcell, Levey, Smith, Abdoli, Abebe and Murray2020; Harris et al., Reference Harris, Davies, Finkelstein, Jha, Donner, Abraham and Zuniga2019). The prevalence of CKD and ESKF has been rising over the last 18 years and is anticipated to double by 2030–2040 (Foreman et al., Reference Foreman, Marquez, Dolgert, Fukutaki, Fullman, McGaughey and Murray2018; Liyanage et al., Reference Liyanage, Ninomiya, Jha, Neal, Patrice, Okpechi and Perkovic2015). The aetiology of CKD and ESKF most commonly involves diabetes (types 1 and 2) and hypertension (Bikbov et al., Reference Bikbov, Purcell, Levey, Smith, Abdoli, Abebe and Murray2020) and, to a lesser extent auto-immune and infectious diseases, environmental pollution (Ekrikpo et al., Reference Ekrikpo, Kengne, Bello, Effa, Noubiap, Salako and Okpechi2018) and genetic conditions (Lunyera et al., Reference Lunyera, Mohottige, Von Isenburg, Jeuland, Patel and Stanifer2016).
About 27% of CKD/ESKF patients have depression (Mosleh et al., Reference Mosleh, Alenezi, Al Johani, Alsani, Fairaq and Bedaiwi2020). Depression amongst CKD/ESKF patients seems to be associated with early initiation of dialysis and an increased mortality compared to those without depression (Farrokhi, Abedi, Beyene, Kurdyak, & Jassal, Reference Farrokhi, Abedi, Beyene, Kurdyak and Jassal2014; Palmer et al., Reference Palmer, Vecchio, Craig, Tonelli, Johnson, Nicolucci and Strippoli2013). Preventing, diagnosing and treating depression in this population is challenging. Research guidance is inconclusive due to small sample sizes, the inclusion of individuals with different aetiologies of disease or types of dialysis, and a lack of appropriate control groups. Evidence for the efficacy of antidepressant medication in patients with CKD/ESKF and depression is poor as these patients are generally excluded from trials due to concerns about safety and adverse events. There are also reports that selective serotonin reuptake inhibitors may not be effective in this population (Hedayati et al., Reference Hedayati, Gregg, Carmody, Jain, Toups, Rush and Trivedi2017) and there is sub-optimal management of antidepressant medication in the dialysis population in the UK (Guirguis et al., Reference Guirguis, Chilcot, Almond, Davenport, Wellsted and Farrington2020).
Inflammation is present in up to 50% of individuals with CKD/ESKF and may increase with the progression of the illness (Rapa, Di Iorio, Campiglia, Heidland, & Marzocco, Reference Rapa, Di Iorio, Campiglia, Heidland and Marzocco2019). There is growing evidence that inflammation may lead to depressive symptoms (DS) and depressive disorders. While comorbidity of CKD and ESKF with depression is evident, it is unclear whether inflammation is more prevalent in individuals with depression and CKD/ESKF individuals compared to individuals with CKD/ESKF, who do not have depression. If proven, such findings would highlight potential benefit of prevention and treatment strategies for depression that targets inflammation. Clinicians need better guidance on treating depression in ESKF patients, taking account of comorbidities and associated inflammation as a contributing factor.
We conducted a systematic review and meta-analysis on inflammation and depression in patients with CKD/ESKF to clarify the evidence base on the relationship between depression and CKD/ESKF and to identify inflammatory biomarkers associated with depression in CKD/ESKF patients.
Methods
The review was conducted in accord with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow and Moher2021), and registered on PROSPERO on 28th August 2019 (CRD42019141305) (Simone Jayakumar, Bhui, Schofield, Ronaldson, Yaqoob, & Carvalho, Reference Simone Jayakumar, Bhui, Schofield, Ronaldson, Yaqoob and Carvalho2019).
Search strategy
A sample search is shown in online Supplementary materials. The search was initially run in May 2019 and re-run in September 2020 and January 2022, where two eligible studies were identified for inclusion. We searched electronic databases (PubMed, Embase, MEDLINE, PsychINFO/PsychArticles, Scopus, Web of Science, CINAHL and Cochrane CENTRAL) for published studies from the inception of database until 19 January 2022, using a search strategy developed with an information scientist. To optimise the capture of relevant research, the first 200 relevant publications from Google Scholar were also considered (Bramer, Rethlefsen, Kleijnen, & Franco, Reference Bramer, Rethlefsen, Kleijnen and Franco2017). Controlled (MESH terms) and text entries were used across all electronic databases (see online Supplementary materials for MESH terms used). Databases such as DARE, ETHOS, OATD, NICE and PROSPERO were checked for existing or ongoing reviews prior to database searches. Forward and backward citation tracking was undertaken for studies that entered the review and progressed to data extraction.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were set out using the Population, Exposure, Comparison, Outcome (PECO) framework (Morgan, Whaley, Thayer, & Schünemann, Reference Morgan, Whaley, Thayer and Schünemann2018). The criteria were developed with clinical and research experts and patient and public involvement (PPI) representatives. The review includes studies of (a) adult patients (⩾18 years) with ESKF or CKD, (b) data on pro- and anti-inflammatory biomarkers and (c) depression measured by standardised clinical interviews, or by administered or self-report validated psychometric instruments. We included case-control, cohort (prospective and retrospective) and cross-sectional studies and non-randomised intervention studies and randomised controlled trials (RCTs). Exclusion criteria included preclinical studies, non-English language studies and studies published in non-peer reviewed journals. Studies investigating the effects of antidepressants or supplements on inflammation, which did not provide baseline data on inflammation in depressed and non-depressed patients were excluded. Given the sparsity of evidence, we excluded studies of kidney transplant or acute kidney injury patients.
Study selection
References, titles and abstracts from electronic database searches were exported into Rayyan QCRI (Ouzzani, Hammady, Fedorowicz, & Elmagarmid, Reference Ouzzani, Hammady, Fedorowicz and Elmagarmid2016). Titles and abstracts were screened independently by two reviewers (SJa and SJe). Screening of titles and abstracts against the inclusion and exclusion criteria was piloted on the first 100 abstracts and revealed that in 50% of publications, information on inflammatory biomarkers was not mentioned in the abstract. Therefore, abstracts meeting all other inclusion criteria underwent full text screening. Full text articles were checked independently by SJa and SJe against the inclusion and exclusion criteria. Authors of studies and potentially relevant conference abstracts were contacted to secure full text articles. After 1 month to respond, one re-contact was attempted. Inter-rater reliability for the full-text screening indicated good agreement between reviewers (κ: 0.89). Uncertainties were resolved by consensus and unresolved discrepancies were referred to a third reviewer (KB).
Data extraction
A data extraction table was piloted on 10 studies and refined (see online Supplementary materials for data extraction fields). Study data were extracted independently by two reviewers (SJa and SJe) and charted in an Excel spreadsheet. Study characteristics extracted included study authors, year published, sample description, methodological characteristics, demographics (age, gender, ethnicity), depression definition and cut-off scores, type and levels of inflammatory biomarkers and main results. Statistical information extracted included means/medians, standard deviations/range/interquartile range for outcome and exposure of interest, N for depressed and non-depressed groups, test-statistic and p values. Discrepancies were discussed and resolved between the two reviewers (95.2% agreement). If data were not available on the outcome/exposure of interest, then corresponding authors were contacted and those that responded within a month were included in the meta-analysis.
Methodological quality and risk of bias
The risk of bias was assessed independently by two reviewers (SJe and SJa). Study quality and risk of bias are presented in online Supplementary materials. RCTs were assessed using the ‘Risk of Bias 2’ (RoB 2) (Sterne et al., Reference Sterne, Savović, Page, Elbers, Blencowe, Boutron and Higgins2019); non-randomised interventional studies (NRIS) were assessed using the ‘Risk Of Bias In Non-randomized Studies – of Interventions’ (ROBINS-I) (Sterne et al., Reference Sterne, Hernán, Reeves, Savović, Berkman, Viswanathan and Higgins2016).
The Newcastle–Ottawa Scale was used to assess the quality of cohort studies (CS) (Wells et al., Reference Wells, Shea, O Connell, Peterson, Welch, Losos and Petersen2014). Study quality is rated as good, fair or poor across selection, comparability and exposure categories. Studies were awarded 1 point for ascertainment of exposure if information was provided on how inflammatory biomarkers were measured. For assessment of outcome, studies were awarded 1 point if depression was diagnosed through a structured clinical interview or if data were obtained from medical records; no points were allocated for self-report measures or no description.
Cross-sectional studies (CSSs) were assessed using the AXIS tool designed specifically for CSSs (Downes, Brennan, Williams, & Dean, Reference Downes, Brennan, Williams and Dean2016). The tool does not provide a numerical score but allows the reviewer to assess each individual aspect of study design based on to give an overall judgement of study quality. CS from which cross-sectional data from one time-point were used, were assessed using the AXIS tool.
Data analysis
Where number of studies permitted a meta-analysis was conducted, otherwise, a narrative synthesis of the studies was undertaken (overview of narrative synthesis is presented in online Supplementary materials).
Meta-analysis
Studies providing baseline or longitudinal data on cytokine levels in patients with or without depression were included in the meta-analysis and in bivariate subgroup analysis. A minimum of three studies per inflammatory marker were considered sufficient for meta-analysis (Ryan, Reference Ryan2016). For continuous data outcomes a weighted mean difference and 95% confidence intervals (CIs) were calculated using a random effects model which also allows suspected heterogeneity between studies (Higgins et al., Reference Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch2019). Forest plots were generated, and pooled effect sizes were calculated using Comprehensive Meta-Analysis (CMA, version 3) (Borenstein, Hedges, Higgins, & Rothstein, Reference Borenstein, Hedges, Higgins and Rothstein2009). The standardised mean difference (SMD) was computed from studies providing means and standard deviations (s.d.) for cytokine levels in patients with or without depression. Where required s.d. were calculated from CIs or standard errors (s.e.) using a verified formula (Wan, Wang, Liu, & Tong, Reference Wan, Wang, Liu and Tong2014). If a study provided a median, interquartile range (including the first and third quartiles) and sample size, this was converted into an estimated sample mean and s.d. using a formula (Wan et al., Reference Wan, Wang, Liu and Tong2014). Where studies provided a correlation coefficient and a sample size instead of a mean and s.d., this was entered into CMA to compute the effect size. Where studies did not provide a mean and s.d. or correlation coefficient a standardised or non-standardised regression coefficient with s.d. and sample sizes for patients with or without depression with 95% CIs, were entered into CMA to compute the effect size (Wilson, Reference Wilson2001). All effect sizes were calculated such that positive values demonstrate higher levels of inflammatory markers in depressed patients, and negative values indicate the opposite. Where adequate data were not provided, study authors were contacted for additional data and were given 3 weeks to respond. Ten per cent (5/50) of authors contacted provided the additional data required for the study to be included in the meta-analysis.
Quality check
To investigate heterogeneity, subgroup and sensitivity analysis were carried out for all inflammatory markers. Within group heterogeneity was assessed using Higgins I 2 with a ⩾50% cut-off for ‘substantial heterogeneity’ (Wan et al., Reference Wan, Wang, Liu and Tong2014). Cochrane's Q with p values <0.05 was also reported to indicate significant within and between group heterogeneity. Likely sources of heterogeneity investigated included the way in which depression was defined (structured clinical interview or validated self-report depression tool) and the type of data provided by studies which was used to calculate the effect size. Sensitivity analyses was carried out on study design for each inflammatory marker (cross-sectional; longitudinal; RCT) and study quality (poor; fair; good). Currently there is no gold standard method to analyse inflammatory markers. The majority of the studies used either enzyme-linked immunosorbent assay, multiplex or nephelometric methods.
Meta-regression investigating age, gender and ethnicity was considered for inflammatory markers with more than 10 studies; however, these data were reported for the total sample rather than for patients with or without depression rendering it inappropriate (Wan et al., Reference Wan, Wang, Liu and Tong2014). Publication bias using Egger's test for funnel plot symmetry was assessed for inflammatory makers containing more than 10 studies [C-reactive protein (CRP); interleukin-6 (IL-6); tumour necrosis factor-alpha (TNF-α)]. In all analyses, statistical significance was set at p ⩽ 0.05.
Results
A meta-analysis was conducted in a search which yielded 9001 citations (see PRISMA, Fig. 1). Fifty-three studies met our inclusion criteria, and seven additional studies were identified by citation tracking: 60 entered the review.

Fig. 1. PRISMA flow chart of study selection.
Study characteristics
Studies which are included in the review are outlined in online Supplementary Table S1. One study used an RCT design and reported depression severity in comparison with controls (Zhao, Ma, Yang, & Xiao, Reference Zhao, Ma, Yang and Xiao2017). Three were cohort (CS), and 56 were cross-sectional (CSS). Most studies included HD patients (32/60), followed by mixed sample of HD and PD patients (11/60), PD patients (12/60), CKD patients (3/60) and mixed sample of CKD and HD or PD patients (2/60).
Majority of studies used a self-report depression scale to measure the presence of DS or severity (52/60). The self-report depression scales used by studies included the Hospital and Anxiety Depression Scale (Depression subscale) (HADS-D), Beck's Depression Inventory (BDI), Hamilton Depression Rating Scale (HAM-D), Mental Health Inventory (MHI), Geriatric Depression Scale (GDS), Patient Health Questionnaire (PHQ), Center for Epidemiologic Studies Depression Scale (CES-D), Cognitive Depression Index (CDI), Zung Self-Rating Depression Scale (SDS) and Allgemeine Deperessionskala-Langform (ADS-L).
CRP was measured most often (53/60), followed by IL-6 (25/60), TNF-α (12/60), IL-10 (7/60) and a range of other biomarkers (IL-1β, fibrinogen, IL-17, IL-18 and IL-12p70); (note hs-CRP and CRP are referred to as CRP). Thirty (30/60) specifically aimed at investigating the association between inflammatory markers and depression in CKD/ESKF patients or an intervention and are referred to as ‘fit for purpose’ (online Supplementary Table S1). Forty-eight per cent of studies (29/60) reported that patients with active infections and/or inflammatory illnesses were excluded. Seventeen per cent of studies (10/60) excluded patients on anti-inflammatory medication; majority of studies did not report this information. Study quality is presented in online Supplementary Table S2.
Cross-sectional meta-analyses
Meta-analysis of cross-sectional associations between CRP and depression
Fifty-one studies analysed cross-sectional data on major depression (MD) or DS and CRP levels (8370 patients). Overall, there was significantly elevated level of CRP in those with depression compared to those without depression [SMD = 0.50 (95% CI 0.28–0.72); p < 0.0001] (Fig. 2). There was significant heterogeneity (I 2 = 95%; χ2 = 1046.47, df = 50; p < 0.0001; τ 2 = 0.76). Seven studies (7/51) used a structured clinical interview to diagnose MD, while most studies (44/51) used a self-report screening tool to measure DS. Amongst these seven studies (613 patients) which used structured clinical interview to diagnose depression, overall CRP levels had only a trend significance between MD and non-MD groups [SMD = 0.56 (95% CI −0.043 to 1.16); p = 0.08], with moderate significant heterogeneity (I 2 = 55%; χ2 = 13.44, df = 6; p = 0.037; τ 2 = 0.07) (online Supplementary Table S3) (Armaly et al., Reference Armaly, Farah, Jabbour, Bisharat, Qader, Saba and Bowirrat2012; Atalay et al., Reference Atalay, Solak, Biyik, Biyik, Yeksan, Uguz and Turk2010; Choi et al., Reference Choi, Seo, Yoon, Lee, Kim, Lee and Koo2013; Cilan et al., Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007; Kalender, Ozdemir, & Koroglu, Reference Kalender, Ozdemir and Koroglu2006; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016). Four studies (4/7) were fit for purpose. Numbers of MD patients included from all studies were small and ranged from 10 to 47 individuals.

Fig. 2. Random effects meta-analysis forest plot of all studies with baseline CRP levels in depressed and non-depressed patients. Heterogeneity: τ 2 = 0.76, Q-value = 1046.47, df(Q) = 50, I 2 = 95.22%, p ⩽ 0.0001. Total between-group heterogeneity (clinical interview v. self-report): Q-value = 0.47, df(Q) = 1, p = 0.83.
Amongst studies which used self-report tools as diagnostic for DS, 44 analyses of (from 40 studies; 7757 patients) found significantly higher levels of CRP in the group with DS compared to no DS group [SMD = 0.49 (95% CI 0.25–0.73); p < 0.001] (Armaly et al., Reference Armaly, Farah, Jabbour, Bisharat, Qader, Saba and Bowirrat2012; Atalay et al., Reference Atalay, Solak, Biyik, Biyik, Yeksan, Uguz and Turk2010; Barros, Costa, Mottin, & D'Avila, Reference Barros, Costa, Mottin and D'Avila2016; Bornivelli, Aperis, Giannikouris, Paliouras, & Alivanis, Reference Bornivelli, Aperis, Giannikouris, Paliouras and Alivanis2012; Bossola, Di Stasio, Giungi, Rosa, & Tazza, Reference Bossola, Di Stasio, Giungi, Rosa and Tazza2015; Bossola et al., Reference Bossola, Ciciarelli, Di Stasio, Conte, Vulpio, Luciani and Tazza2010; Boulware et al., Reference Boulware, Liu, Fink, Coresh, Ford, Klag and Powe2006; Chilcot et al., Reference Chilcot, Friedli, Guirguis, Wellsted, Farrington and Davenport2017; Choi et al., Reference Choi, Seo, Yoon, Lee, Kim, Lee and Koo2013; Cilan et al., Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Dogan, Erkoc, Eryonucu, Sayarlioglu, & Agargun, Reference Dogan, Erkoc, Eryonucu, Sayarlioglu and Agargun2005; Dong et al., Reference Dong, Pi, Xiong, Liao, Hao, Liu and Zheng2016; Fan et al., Reference Fan, Sarnak, Tighiouart, Drew, Kantor, Lou and Weiner2014; Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Güney et al., Reference Güney, Biyik, Yeksan, Biyik, Atalay, Solak and Türk2008; Gyamlani et al., Reference Gyamlani, Basu, Geraci, Lee, Moxey, Clark and Dubbert2011; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Hsu et al., Reference Hsu, Chen, Hsiao, Wu, Sun, Chou and Wu2009a, Reference Hsu, Chen and Wu2009b; Hung et al., Reference Hung, Wu, Chen, Ma, Tseng, Yang and Lu2011; Jong et al., Reference Jong, Tsai, Lin, Ma, Guo, Hung and Hung2017; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007, Reference Kalender, Ozdemir and Koroglu2006; Kim, Kim, Kim, & Song, Reference Kim, Kim, Kim and Song2012; Ko et al., Reference Ko, Kim, Yu, Jo, Cho and Kim2010; Kusztal et al., Reference Kusztal, Trafidło, Madziarska, Augustyniak-Bartosik, Karczewski, Weyde and Klinger2018; Li et al., Reference Li, An, Mao, Wei, Chen, Yang and Yu2011; Malhotra et al., Reference Malhotra, Persic, Zhang, Brown, Tao, Rosales and Kotanko2017; Micozkadioglu et al., Reference Micozkadioglu, Micozkadioglu, Zumrutdal, Erdem, Ozdemir, Sezer and Haberal2006; Mok et al., Reference Mok, Liu, Lam, Kwan, Chan, Ma and Chan2019; Nie et al., Reference Nie, Wang, Zhang, Xiong, Liao, Hao and Dong2019; Nowak, Adamczak, & Więcek, Reference Nowak, Adamczak and Więcek2013; Oguz et al., Reference Oguz, Akoglu, Ulusal Okyay, Yayar, Karaveli Gursoy, Buyukbakkal and Ayli2016; Park et al., Reference Park, Lee, Lee, Kim, Oh, Joo and Oh2012, Reference Park, Yoon, Son, Jung, Joo, Chin and Oh2010; Schricker et al., Reference Schricker, Heider, Schanz, Dippon, Alscher, Weiss and Kimmel2019; Simic Ogrizovic et al., Reference Simic Ogrizovic, Jovanovic, Dopsaj, Radovic, Sumarac, Bogavac and Nesic2009; Su et al., Reference Su, Ng, Huang, Chi, Lee, Lai and Lee2012; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012; Tufan, Yıldız, Dogan, Yıldız, & Sevinir, Reference Tufan, Yıldız, Dogan, Yıldız and Sevinir2015; Uglešić et al., Reference Uglešić, Ljutić, Lasić, Jeličić, Višić, Glavina and Meter2015; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016; Yavuz et al., Reference Yavuz, Yavuz, Altunoglu, Canoz, Sezer, Yalcin and Demirag2015; Zhang et al., Reference Zhang, Zhang, Ni, Bao, Huang, Wu and Chen2014). Significant heterogeneity was detected (I 2 = 96%; χ2 = 1032.71, df = 43; p < 0.0001; τ 2 = 0.79). There were no between-group differences when comparison only retained studies using structured clinical interviews for MD or a validated self-report measure of DS (Q-value = 0.47, df = 1; p = 0.83). There was no difference for total between-group heterogeneity when comparing effect size calculation formats (online Supplementary Table S5) or study quality (online Supplementary Table S6). There was no difference for total between-group heterogeneity when comparing studies that excluded patients with active infections/inflammatory diseases (online Supplementary Table S7) or non-steroidal anti-inflammatory drugs (NSAIDs). However, significantly higher levels of CRP were reported in patients with depression for studies that did not exclude patients on NSAIDs compared to those that did (online Supplementary Table S8).
Meta-analysis of cross-sectional associations between IL-6 and depression
Twenty-nine analyses from 27 studies (5140 patients) were appropriate for inclusion in the meta-analyses investigating cross-sectional associations between IL-6 and depression (Alshogran, Khalil, Oweis, Altawalbeh, & Alqudah, Reference Alshogran, Khalil, Oweis, Altawalbeh and Alqudah2018; Bossola et al., Reference Bossola, Ciciarelli, Di Stasio, Conte, Vulpio, Luciani and Tazza2010, Reference Bossola, Di Stasio, Giungi, Rosa and Tazza2015; Boulware et al., Reference Boulware, Liu, Fink, Coresh, Ford, Klag and Powe2006; Brys et al., Reference Brys, Di Stasio, Lenaert, Sanguinetti, Picca, Calvani and Bossola2020; Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Damayanti, Nasution, & Lubis, Reference Damayanti, Nasution and Lubis2018; Dervisoglu, Kir, Kalender, Eraldemir, & Caglayan, Reference Dervisoglu, Kir, Kalender, Eraldemir and Caglayan2008; Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Hung et al., Reference Hung, Wu, Chen, Ma, Tseng, Yang and Lu2011; Jong et al., Reference Jong, Tsai, Lin, Ma, Guo, Hung and Hung2017; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007; Knuth et al., Reference Knuth, Radtke, Rocha, da Silva, Dalsóglio, Gazal and Oses2014; Kusztal et al., Reference Kusztal, Trafidło, Madziarska, Augustyniak-Bartosik, Karczewski, Weyde and Klinger2018; Nie et al., Reference Nie, Wang, Zhang, Xiong, Liao, Hao and Dong2019; Nowak et al., Reference Nowak, Adamczak and Więcek2013; Schricker et al., Reference Schricker, Heider, Schanz, Dippon, Alscher, Weiss and Kimmel2019; Simic Ogrizovic et al., Reference Simic Ogrizovic, Jovanovic, Dopsaj, Radovic, Sumarac, Bogavac and Nesic2009; Sonikian et al., Reference Sonikian, Metaxaki, Papavasileiou, Boufidou, Nikolaou, Vlassopoulos and Vlahakos2010; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012; Uglešić et al., Reference Uglešić, Ljutić, Lasić, Jeličić, Višić, Glavina and Meter2015; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016; Zhao et al., Reference Zhao, Ma, Yang and Xiao2017). Overall, there was significantly higher levels of IL-6 in patients with depression compared to patients without depression [SMD = 0.67 (95% CI 0.35–0.99); p < 0.001]. There was significant heterogeneity [I 2 = 96%; χ2 = 696.72, df = 28; p < 0.001; τ 2 = 0.71) (Fig. 3). Four studies (317 patients) used a structured clinical interview to diagnose MD. Amongst these, no significant differences in IL-6 levels were found between MD and non-MD groups [SMD = 0.23 (95% CI −0.66 to 1.12); p = 0.62] (Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016); and no significant heterogeneity (I 2 = 0%; χ2 = 1.10, df = 3; p = 0.78; τ 2 = 0.00). Sample sizes of patients with depression were very small in all (9–11 people with MD) (Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007). Amongst studies which used self-report DS, 25 analyses (from 22 studies; 4823 patients) found significant differences in IL-6 levels in individuals with increased DS compared to no DS (SMD = 0.74, 95% CI −0.39 to 1.08, p < 0.001) (Alshogran et al., Reference Alshogran, Khalil, Oweis, Altawalbeh and Alqudah2018; Bossola et al., Reference Bossola, Ciciarelli, Di Stasio, Conte, Vulpio, Luciani and Tazza2010, Reference Bossola, Di Stasio, Giungi, Rosa and Tazza2015; Boulware et al., Reference Boulware, Liu, Fink, Coresh, Ford, Klag and Powe2006; Brys et al., Reference Brys, Di Stasio, Lenaert, Sanguinetti, Picca, Calvani and Bossola2020; Damayanti et al., Reference Damayanti, Nasution and Lubis2018; Dervisoglu et al., Reference Dervisoglu, Kir, Kalender, Eraldemir and Caglayan2008; Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Hung et al., Reference Hung, Wu, Chen, Ma, Tseng, Yang and Lu2011; Jong et al., Reference Jong, Tsai, Lin, Ma, Guo, Hung and Hung2017; Knuth et al., Reference Knuth, Radtke, Rocha, da Silva, Dalsóglio, Gazal and Oses2014; Kusztal et al., Reference Kusztal, Trafidło, Madziarska, Augustyniak-Bartosik, Karczewski, Weyde and Klinger2018; Nie et al., Reference Nie, Wang, Zhang, Xiong, Liao, Hao and Dong2019; Nowak et al., Reference Nowak, Adamczak and Więcek2013; Schricker et al., Reference Schricker, Heider, Schanz, Dippon, Alscher, Weiss and Kimmel2019; Simic Ogrizovic et al., Reference Simic Ogrizovic, Jovanovic, Dopsaj, Radovic, Sumarac, Bogavac and Nesic2009; Sonikian et al., Reference Sonikian, Metaxaki, Papavasileiou, Boufidou, Nikolaou, Vlassopoulos and Vlahakos2010; Taraz et al., Reference Taraz, Khatami, Dashti-Khavidaki, Akhonzadeh, Noorbala, Ghaeli and Taraz2013; Uglešić et al., Reference Uglešić, Ljutić, Lasić, Jeličić, Višić, Glavina and Meter2015; Zhao et al., Reference Zhao, Ma, Yang and Xiao2017). All 25 analyses showed significant considerable heterogeneity (I 2 = 97%; χ2 = 692.92, df = 24; p < 0.001; τ 2 = 0.75) (online Supplementary Table S3). Comparison of total between-group heterogeneity comparing structured clinical interviews with depression defined using a validated self-report measure reported no significant heterogeneity (χ2 = 1.08, df = 1; p = 0.30).

Fig. 3. Random effects meta-analysis forest plot of all studies with baseline IL-6 levels in depressed and non-depressed patients. Heterogeneity: τ 2 = 0.71, Q-value = 696.719, df(Q) = 28, I 2 = 95.98%, p ⩽ 0.001. Total between-group heterogeneity (clinical interview v. self-report): Q-value = 1.08, df(Q) = 1, p = 0.30.
Significantly higher IL-6 levels in depressed group were found irrespective of how effect sizes were calculated and there was significant heterogeneity (online Supplementary Table S5). Regardless of study design, both cross-sectional and RCT data reported significantly higher IL-6 levels in MD/DS compared to non-MD/DS groups (online Supplementary Table S4). Significant considerable heterogeneity was reported for CSSs but not for RCTs; furthermore total between-group heterogeneity was found to be significant when comparing cross-sectional to RCTs. Only good quality CSSs (21 analyses) reported significantly higher levels of IL-6 in depressed compared to non-depressed groups (online Supplementary Table S4) (Alshogran et al., Reference Alshogran, Khalil, Oweis, Altawalbeh and Alqudah2018; Bossola et al., Reference Bossola, Ciciarelli, Di Stasio, Conte, Vulpio, Luciani and Tazza2010, Reference Bossola, Di Stasio, Giungi, Rosa and Tazza2015; Brys et al., Reference Brys, Di Stasio, Lenaert, Sanguinetti, Picca, Calvani and Bossola2020; Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Damayanti et al., Reference Damayanti, Nasution and Lubis2018; Dervisoglu et al., Reference Dervisoglu, Kir, Kalender, Eraldemir and Caglayan2008; Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Hung et al., Reference Hung, Wu, Chen, Ma, Tseng, Yang and Lu2011; Jong et al., Reference Jong, Tsai, Lin, Ma, Guo, Hung and Hung2017; Knuth et al., Reference Knuth, Radtke, Rocha, da Silva, Dalsóglio, Gazal and Oses2014; Kusztal et al., Reference Kusztal, Trafidło, Madziarska, Augustyniak-Bartosik, Karczewski, Weyde and Klinger2018; Nie et al., Reference Nie, Wang, Zhang, Xiong, Liao, Hao and Dong2019; Nowak et al., Reference Nowak, Adamczak and Więcek2013; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012; Uglešić et al., Reference Uglešić, Ljutić, Lasić, Jeličić, Višić, Glavina and Meter2015; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016). These results were not replicated in fair quality studies (Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007; Schricker et al., Reference Schricker, Heider, Schanz, Dippon, Alscher, Weiss and Kimmel2019; Simic Ogrizovic et al., Reference Simic Ogrizovic, Jovanovic, Dopsaj, Radovic, Sumarac, Bogavac and Nesic2009; Sonikian et al., Reference Sonikian, Metaxaki, Papavasileiou, Boufidou, Nikolaou, Vlassopoulos and Vlahakos2010); both fair and good quality studies reported considerable heterogeneity. There was no difference for total between-group heterogeneity when comparing studies that excluded patients with active infections/inflammatory diseases (online Supplementary Table S7) or NSAIDs. However, significantly higher levels of IL-6 were reported in patients with depression for studies that did not exclude patients on NSAIDs compared to those that did (online Supplementary Table S8).
Meta-analysis of cross-sectional associations between TNF-α and MD/DS
Eleven analyses from 11 studies (1838 patients) were included in the meta-analyses (Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Dervisoglu et al., Reference Dervisoglu, Kir, Kalender, Eraldemir and Caglayan2008; Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Jong et al., Reference Jong, Tsai, Lin, Ma, Guo, Hung and Hung2017; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007; Ko et al., Reference Ko, Kim, Yu, Jo, Cho and Kim2010; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016). Overall, these studies found a trend for higher TNF-α levels in the MD/DS group compared to the non-MD/DS group [SMD = 0.38 (95% CI −0.02 to 0.78); p = 0.07]. All 11 analyses showed significant heterogeneity (I 2 = 93%; χ2 = 139.03, df = 10; p < 0.001; τ 2 = 0.4) (Fig. 4).
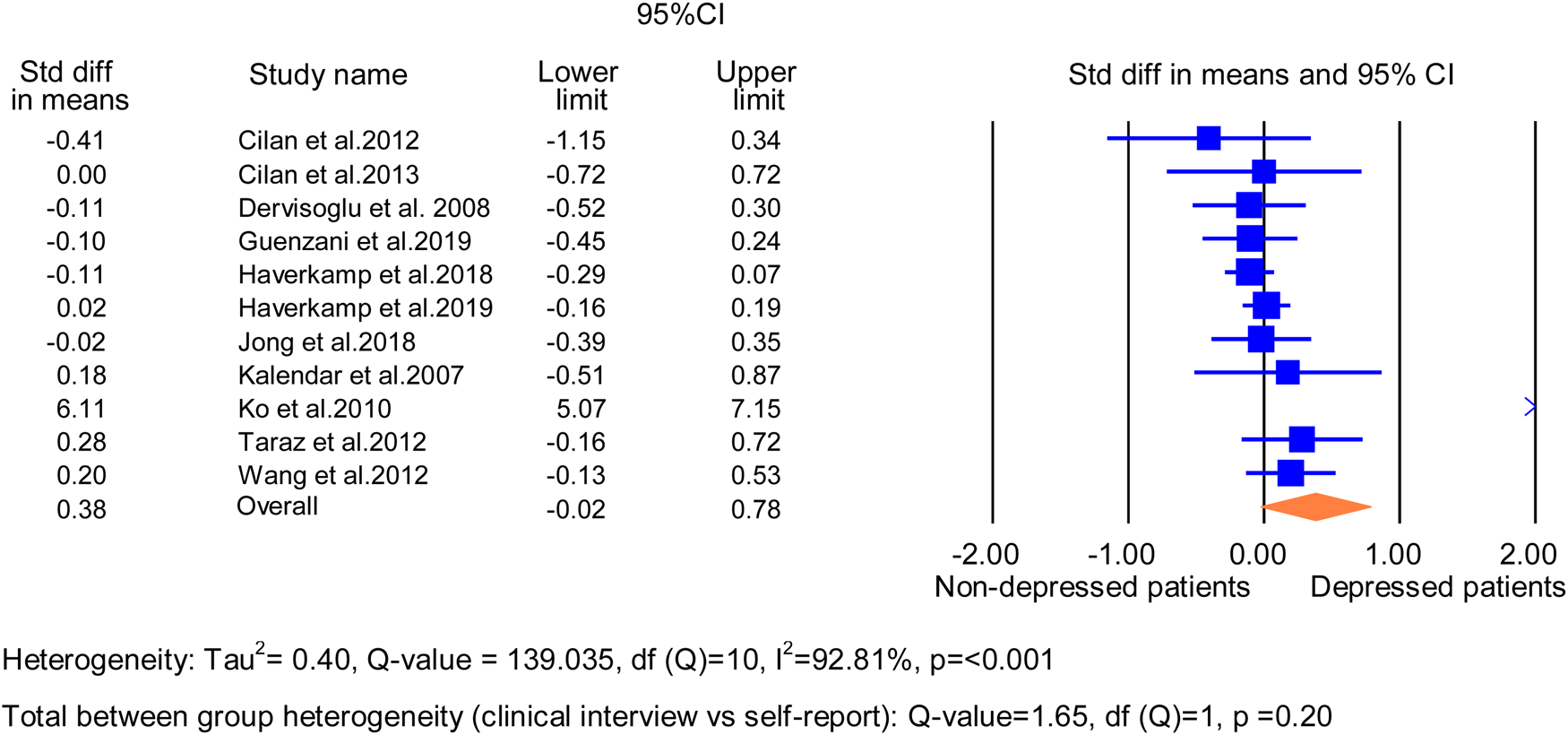
Fig. 4. Random effects meta-analysis forest plot of all studies with baseline TNF-α levels in depressed and non-depressed patients. Heterogeneity: τ 2 = 0.40, Q-value = 139.035, df(Q) = 10, I 2 = 92.81%, p ⩽ 0.001. Total between-group heterogeneity (clinical interview v. self-report): Q-value = 1.65, df(Q) = 1, p = 0.20.
Four studies (317 patients) used a structured clinical interview to diagnose MD. These found no significant differences in TNF-α levels between the MD and non-MD groups [SMD = 0.01 (95% CI −0.72 to 0.73); p = 0.99] (Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007; Wang et al., Reference Wang, Liu, Lian, Li, Liu and Li2016). Four analyses showed no significant differences in heterogeneity (I 2 = 0%; χ2 = 2.22, df = 3; p = 0.53; τ 2 = 0.00) (online Supplementary Table S3). The sample sizes of depressed patients were very small in all (9–11 MD individuals) (Cilan et al., Reference Cilan, Oguzhan, Unal, Turan, Koc, Sipahioglu and Oymak2012, Reference Cilan, Sipahioglu, Oguzhan, Unal, Turan, Koc and Oymak2013; Kalender et al., Reference Kalender, Dervisoglu, Sengul, Ozdemir, Akhan, Yalug and Uzun2007) but in one study (Wang et al., Reference Wang, Wu, Hsu, Wu, Sun, Chou and Chen2012).
Amongst studies which used self-report DS (1521 patients), there was significantly higher levels of TNF in the high DS group compared to the no DS [SMD = 0.59 (95% CI 0.07–1.12); p = 0.03] (Dervisoglu et al., Reference Dervisoglu, Kir, Kalender, Eraldemir and Caglayan2008; Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Jong et al., Reference Jong, Tsai, Lin, Ma, Guo, Hung and Hung2017; Ko et al., Reference Ko, Kim, Yu, Jo, Cho and Kim2010; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012). All seven analyses showed significant heterogeneity (I 2 = 96%; χ2 = 136.58, df = 6; p < 0.0001; τ 2 = 0.51) (online Supplementary Table S3).
Comparison of studies defining depression using structured clinical interviews with those using a validated self-report measure reported no significant heterogeneity (χ2 = 1.65; df = 1; p = 0.20). The type of effect size did not account for the findings (online Supplementary Table S5). Both fair and good quality studies reported non-significant findings in TNF-α levels between the MD/DS and non-MD/DS groups with significant heterogeneity reported for good quality studies (online Supplementary Table S4). There was no significant total between-group heterogeneity when comparing studies that excluded patients with active infections/inflammatory diseases or NSAIDs. Studies excluding patients with infections/inflammatory diseases reported significantly higher levels of TNF-α in depressed patients compared to non-depressed patients (online Supplementary Tables S7 and S8).
Meta-analysis of cross-sectional associations between IL-10 and depression
Six analyses from six studies (1427 patients) were appropriate for inclusion in the meta-analyses investigating cross-sectional associations between IL-10 and depression (Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Ko et al., Reference Ko, Kim, Yu, Jo, Cho and Kim2010; Simic Ogrizovic et al., Reference Simic Ogrizovic, Jovanovic, Dopsaj, Radovic, Sumarac, Bogavac and Nesic2009; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012) (Fig. 5). All used a validated self-report measure to define the high DS group. IL-10 levels were significantly lower in the DS compared to the non-DS group [SMD = −0.57 (95% CI −1.09 to 0.06); p < 0.0001]. All six analyses showed significant heterogeneity (I 2 = 95%; χ2 = 95.02; df = 5; p < 0.0001; τ 2 = 0.38). Effect size calculation format showed no effect on the findings; however, significant heterogeneity was reported only for effect size calculations using means and s.d. (online Supplementary Table S5). Only good quality studies (five analyses) reported significantly lower levels of IL-10 in depressed compared to non-depressed groups (online Supplementary Table S4) (Guenzani et al., Reference Guenzani, Buoli, Caldiroli, Carnevali, Serati, Vezza and Vettoretti2019; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018, Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Ko et al., Reference Ko, Kim, Yu, Jo, Cho and Kim2010; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012). No significant total between-group heterogeneity reported for studies excluding patients with infections/inflammatory diseases or NSAIDs, compared to those that did not. However, significantly lower levels of IL-10 were reported in studies that excluded patients with infections/inflammatory diseases (online Supplementary Table S7). The opposite was observed in studies that did not exclude patients on NSAIDs, where levels of IL-10 were significantly lower in patients with depression (online Supplementary Table S8).

Fig. 5. Random effects meta-analysis forest plot of all studies with baseline IL-10 levels in depressed and non-depressed patients. Heterogeneity: τ 2 = 0.38, Q-value = 95.024, df(Q) = 5, I 2 = 94.74%, p ⩽ 0.0001. NB: all baseline studies reporting IL-10 levels in depressed and non-depressed patients used a self-report measure.
Meta-analysis of cross-sectional associations between IL-1β and depression
Three analyses from three studies (1086 patients) were appropriate for inclusion in the meta-analysis (Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Honig2018; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019; Taraz et al., Reference Taraz, Khatami, Gharekhani, Abdollahi, Khalili and Dashti-Khavidaki2012) (online Supplementary Fig. S1). All used a validated self-report tool to define DS group. There were no significant differences in IL-1β levels in high compared to the no-DS groups [SMD = −0.01 (95% CI −0.13 to 0.11) p = 0.093]. All three analyses showed no significant heterogeneity (I 2 = 0%; χ2 = 0.98; df = 2; p = 0.61; τ 2 = 0.00) and were of good quality. Effect size calculation format reported no significant influence on reported IL-1β levels in DS compared to no-DS groups (online Supplementary Table S5). There were no significant differences in the levels of IL-1β in studies that excluded patients with infections/inflammatory diseases or NSAIDs, compared to those that did not (online Supplementary Tables S7 and S8).
Meta-analysis of cross-sectional associations between fibrinogen and depression
Four analyses from four studies (297 patients) were appropriate for inclusion (Bossola et al., Reference Bossola, Ciciarelli, Di Stasio, Conte, Antocicco, Rosa and Tazza2012, Reference Bossola, Di Stasio, Giungi, Rosa and Tazza2015; Ko et al., Reference Ko, Kim, Yu, Jo, Cho and Kim2010) (online Supplementary Fig. S2). All used a validated self-report measure to define the DS group. Significantly higher levels of fibrinogen were reported in high DS compared to no-DS groups [SMD = 0.64 (95% CI 0.33–0.95); p < 0.0001]. There was no significant heterogeneity (I 2 = 35%; χ2 = 4.67; df = 3; p = 0.19; τ 2 = 0.036). Effect size calculation format showed no effect (online Supplementary Table S5). No significant total between-group heterogeneity reported for studies excluding patients with infections/inflammatory diseases, compared to those that did not and both groups reported significantly higher levels of fibrinogen in patients with depression compared to patients without (online Supplementary Table S7).
Longitudinal associations between DS and inflammatory cytokines
Narrative synthesis
There were three longitudinal studies which investigated inflammatory markers and future DS (Barros et al., Reference Barros, Costa, Mottin and D'Avila2016; Bossola et al., Reference Bossola, Ciciarelli, Di Stasio, Conte, Antocicco, Rosa and Tazza2012; Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019). There were not sufficient studies for each inflammatory marker for meta-analysis, thus a narrative review was executed.
All studies were similar in terms of dialysis modality (HD patients), depression definition (BDI) and percentage of females (ranging from 39.4% to 41%). There was one fit for purpose study (Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019) that investigated whether higher levels of inflammation were associated with the development of future DS in CKD/ESKF patients (Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019). They found that CRP was an independent predictor for an increase in DS. Patients with higher baseline serum CRP levels, compared to patients with lower serum CRP levels, had increased DS at 12 month, but not at 6 month follow-up (Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019). Haverkamp et al. (Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019) found no significant longitudinal associations between DS, as measured by BDI, and IL-6, TNF-α, IL-10 and IL-1β (Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019). Barros et al. (Reference Barros, Costa, Mottin and D'Avila2016) did not report a change in inflammatory levels and association with worsening depression in HD patients. Bossola et al. (Reference Bossola, Ciciarelli, Di Stasio, Conte, Antocicco, Rosa and Tazza2012) reported that fibrinogen levels are not associated with increased DS at follow-up in HD patients. In cross-sectional analysis, both Barros et al. (Reference Barros, Costa, Mottin and D'Avila2016) and Haverkamp et al. (Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019) reported significantly higher CRP levels in DS patients at the 12 month follow-up compared to the no DS group.
Discussion
Depression is the most prevalent psychosocial factor in patients with CKD/ESKF and is associated with increased morbidity and mortality (Rosenthal Asher, Ver Halen, & Cukor, Reference Rosenthal Asher, Ver Halen and Cukor2012). Accumulating evidence suggests that inflammation may be causal to the development of DS (Khandaker et al., Reference Khandaker, Zuber, Rees, Carvalho, Mason, Foley and Burgess2019). Whether inflammation has a role in depressed CKD/ESKF patient is not yet clear. Higher levels of inflammation may point towards those who may particularly benefit from shared preventive approaches and better inform treatment strategies.
There is one other systematic review and meta-analysis which investigated depression and inflammation in kidney disease (Gregg et al., Reference Gregg, Carmody, Le, Martins, Trivedi and Hedayati2020). The review only included studies with the biomarker albumin as their focus was on protein/energy wasting biomarker and consequent inflammation, thus our studies – despite similarities – differ in inclusion criteria and aim. This is the first systematic review and meta-analysis specifically investigating inflammatory biomarkers in depressed patients with CKD/ESKF. We found evidence of higher levels of pro-inflammatory cytokines and acute inflammatory markers, e.g. CRP, IL-6, TNF, fibrinogen, but not for IL-1β, and lower levels of the anti-inflammatory cytokine IL-10 in CKD patients with increased DS in CSSs. Pooled studies were statistically significant for higher CRP and IL-6, and a trend for TNF in CKD/ESKF patients with MD/DS. There was only a trend significant association for higher levels of CRP and clinical depression, and not for the other inflammatory markers. We believe the lack of association is most likely due to power since only a few studies included patients with MD as assessed by clinical structured interview. Similar results were also reported in an umbrella review of depression and peripheral inflammatory biomarkers in a population without chronic illness (Lee et al., Reference Lee, Lee, Park, Park, Kim, Lee and Fusar-Poli2021). Lee et al. (Reference Lee, Lee, Park, Park, Kim, Lee and Fusar-Poli2021) reported significant association between increased DS and CRP, IL-6 and TNF; however this association was no longer significant with chronic MD patients.
Most studies investigated inflammatory markers using validated self-report tools for DS. Fewer studies, a total of seven for CRP and four for IL-6 and TNF, investigated cross-sectional associations using structured clinical interviews to diagnose depression. Most of these studies had very few patients and did not find associations between inflammatory cytokines and MD. Reason for this is most likely lack of power. Inflammation is observed in ~30% of patients with MD in the absence of chronic physical illness (Miller & Raison, Reference Miller and Raison2016), and in ~50% of patients with ESKF (Cobo, Lindholm, & Stenvinkel, Reference Cobo, Lindholm and Stenvinkel2018). Amongst the few studies which used structured clinical interview to diagnose depression, number of depressed patients were small (range 10–47 for CRP and even fewer for other markers), which would have limited our power to detect associations. More generally, there is an absence of studies that looked at inflammatory markers and MD in CKD. No studies investigated the effect of starting dialysis on inflammation and the association with depression. There is variation between studies on when inflammatory parameters were collected in relation to time of dialysis. It is possible that inflammatory levels vary considerably before, during and after dialysis.
Longitudinal studies are lacking in CKD/ESKF. There were three longitudinal studies of inflammatory markers and DS, and only one particularly investigated whether inflammatory markers at baseline predict development of future DS (Haverkamp et al., Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019). Haverkamp et al. (Reference Haverkamp, Loosman, Schouten, Franssen, Kema, van Diepen and Siegert2019) found a positive association for CRP, but not for other inflammatory markers, and future DS. They also included patients with acute infection, which may have skewed the results. Barros et al. (Reference Barros, Costa, Mottin and D'Avila2016) did not report on longitudinal associations of inflammatory markers at baseline and future DS. In individuals without chronic physical illnesses, there is evidence that chronic inflammation predicts DS (Bell et al., Reference Bell, Kivimaki, Bullmore, Steptoe, Consortium and Carvalho2017; Zalli, Jovanova, Hoogendijk, Tiemeier, & Carvalho, Reference Zalli, Jovanova, Hoogendijk, Tiemeier and Carvalho2016).
Inflammation in patients with depression seems to be particularly important in chronically ill individuals (Nikkheslat et al., Reference Nikkheslat, Zunszain, Horowitz, Barbosa, Parker, Myint and Pariante2015). There is preliminary evidence to show that inflammation is a shared pathway likely causal between depression and cardiovascular disease (CVD) (Khandaker et al., Reference Khandaker, Zuber, Rees, Carvalho, Mason, Foley and Burgess2019). More research is needed to confirm whether inflammation is a shared factor partly explaining the high prevalence of depression in the chronic physically ill, and in CKD/ESKF specifically. It is possible that both CVD and depression are underpinned by one (or more) shared pathophysiological mechanism(s) which manifests as distinct conditions in different organs (e.g. brain and kidneys). Indeed, ESKF commonly arises in patients experiencing hypertension and diabetes; and depressive illness is known to be a comorbid complication of medical disorders. There is the need for higher quality, larger, longitudinal studies for evidence to be conclusive of the role of inflammation in CKD/ESKF patients with depression.
We identified marked heterogeneity in study designs, but overall, the type of outcome measure used (self-report v. structured instrument), duration and method of dialysis, methods to measure effect size appeared to have some influence. There were no studies that reported whether dialysis efficacy or duration was associated with higher inflammation and depression. Future studies should focus on CRP, IL-6 and TNF and more explicitly investigate whether there are comorbid medical conditions and include measures of a range of severity of depressive illness in ESKF. The timing of onset of depressive illness is also important to clarify, as a previous history of depression may predispose to later depression in the face of a medical condition or life event with little specificity for ESKF and related inflammation. Our study could not investigate cause or effect as most studies were conducted cross-sectionally. It is possible that DS triggered increased inflammation. Both directions have been seen in the literature. Indeed, histories and contemporary experiences of adversity and trauma also make depression more likely and raise inflammatory markers. Furthermore, ESKF and dialysis are demanding and may lead to adjustment reactions, and pessimism that may not meet criteria for depression but may reflect other life stressors, such as social isolation, fear of loss and death, concerns about dependents, and uncertainty, not to mention the need for lifetime dialysis and a schedule which is dictated by health needs. This will make enjoyment of everyday life more difficult, for example, taking holidays or travel and risks of new onset conditions complicating an already challenging medical condition. Psychological flexibility and adjustment to the diagnosis and treatment are predictors of better outcomes and might be the mechanism by which therapeutic efforts might be helpful (Iida et al., Reference Iida, Fujimoto, Wakita, Yanagi, Suzuki, Koitabashi and Kurita2020). Indeed, acceptance and commitment therapy encourages psychological flexibility and is being tested under many chronic conditions including cancer survivors, and may have a place in the care of dialysis patients living with uncertainty (Fernandez-Rodriguez, Gonzalez-Fernandez, Coto-Lesmes, & Pedrosa, Reference Fernandez-Rodriguez, Gonzalez-Fernandez, Coto-Lesmes and Pedrosa2020).
Limitations
There is limited evidence and few studies that reported on the same inflammatory markers using similar sample sizes. Longitudinal and experimental studies are needed, testing whether inflammation is a shared factor for the development of depression and chronic physical illnesses which could be a target for prevention. Many studies reported here were not fit for purpose, not designed to evaluate our research question. We acknowledge that some medications taken for CKD or related conditions may have anti-inflammatory properties (Salazar, Ennis, & Koh, Reference Salazar, Ennis and Koh2016; Silva, de Figueiredo, & Rios, Reference Silva, de Figueiredo and Rios2019; Webster, Nagler, Morton, & Masson, Reference Webster, Nagler, Morton and Masson2017), which could have influenced the results of our study. As studies included in this review did not specify which medications were taken their effect on the levels of inflammatory marker is unknown. In any case, this bias would contribute to findings towards the null, which was not what we observed here.
It is still unknown whether inflammation is particularly associated with a particular aetiology of CKD/ESKF and depression. There is a need for psychological interventions, social support and anti-inflammatory drugs to investigate its effects on inflammation to observe whether inflammation could be a mechanism. Larger multicentre studies are needed for focusing on specific patient groups, and those with specific comorbidities (Kiecolt-Glaser, Derry, & Fagundes, Reference Kiecolt-Glaser, Derry and Fagundes2015). Finally, it is important to mention in this context directionality of the relationship. As most studies presented here were cross-sectional it is still unclear in the CKD/ESKF population whether inflammation is indeed causally related to development of DS. It is equally possible that inflammation follows DS.
In conclusion, we found evidence for a cross-sectional association of higher levels of pro-inflammatory cytokines and DS or clinical depression in patients with CKD/ESKF. Research is needed to investigate whether inflammation is a target for prevention and treatment of CKD/ESKF patients with depression.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291722003099.
Acknowledgements
We thank Jessica Schofield for her assistance in full text and abstract screening. We thank Fiona Loud and Brian Gracey who commented on the review protocol and were part of the original funding application for the larger programme of work. We also thank the authors who provided full text access and further data to the papers included in the review. We thank colleagues who provided comments on the manuscript: Roisin Mooney and Georgina Hosang. We are grateful to Barts Charity for the original funding and for an extension to address COVID restrictions on recruitment during the pandemic.
Author contributions
Simone Jayakumar (SJa), a Ph.D. candidate, with Stacey Jennings (SJe), undertook searches, screening and extraction under supervision by Bhui (KB), Carvalho and Hosang. SJa conducted the meta-analysis and produced an early draft. Christophe Clesse and SJe contributed to drafting and editing the manuscript. Kristoffer Halvorsrud gave methodological advice and critically assessed the meta-analysis. All authors contributed to the commenting on the manuscript, interpretation of the results and revisions of the manuscript. Livia A. Carvalho, Muhammad Magdi Yaqoob and KB were responsible for the conception, design of the original larger programme, the review, interpretation, supervision and writing of the manuscript. All authors have approved the final version.
Financial support
Barts Charity Grant Reference Number: MGU0428, 21st May 2018. Award to Kamaldeep Bhui (chief-principal investigator, lead on mental health), Carvalho (co-investigator, lead on immune markers), Magdi Yaqoob (co-investigator), Karl Marlowe (co-investigator). Collaborators: Raj Thuraisingham and Andrea Cove. PPI partners: Fiona Loud and Brian Gracey. Kristoffer Halvorsrud is funded in full by the National Institute for Health Research ARC North Thames; there are no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years; there are no other relationships or activities that could appear to have influenced the submitted work. The views expressed in this publication are that of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Conflict of interest
None declared.








